Answered step by step
Verified Expert Solution
Question
1 Approved Answer
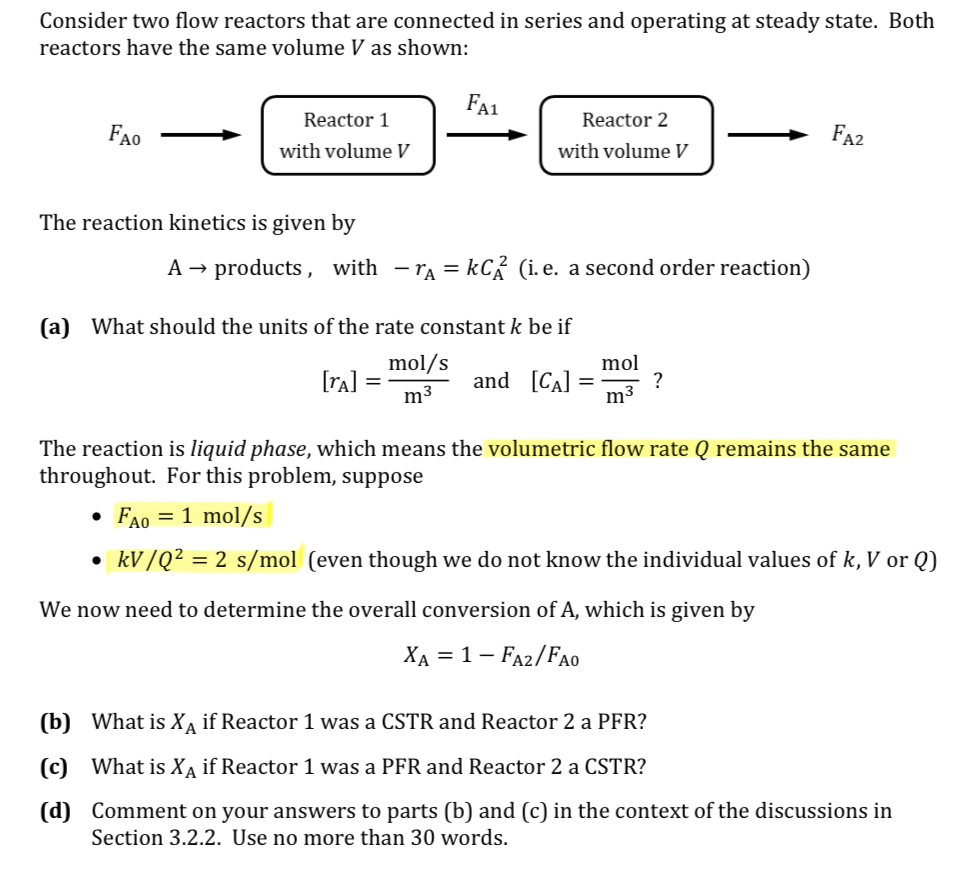
Consider two flow reactors that are connected in series and operating at steady state. Both reactors have the same volume V as shown: The reaction
Consider two flow reactors that are connected in series and operating at steady state. Both reactors have the same volume as shown:
The reaction kinetics is given by
products, with a second order reaction
a What should the units of the rate constant be if
and
The reaction is liquid phase, which means the volumetric flow rate remains the same throughout. For this problem, suppose
even though we do not know the individual values of or
We now need to determine the overall conversion of which is given by
b What is if Reactor was a CSTR and Reactor a PFR
c What is if Reactor was a PFR and Reactor a CSTR
d Comment on your answers to parts b and c in the context of the discussions in Section Use no more than words.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


