Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider two systems, one consisting of 8 lattice sites and containing 3 atoms and the other consisting of 6 lattice sites and containing 2
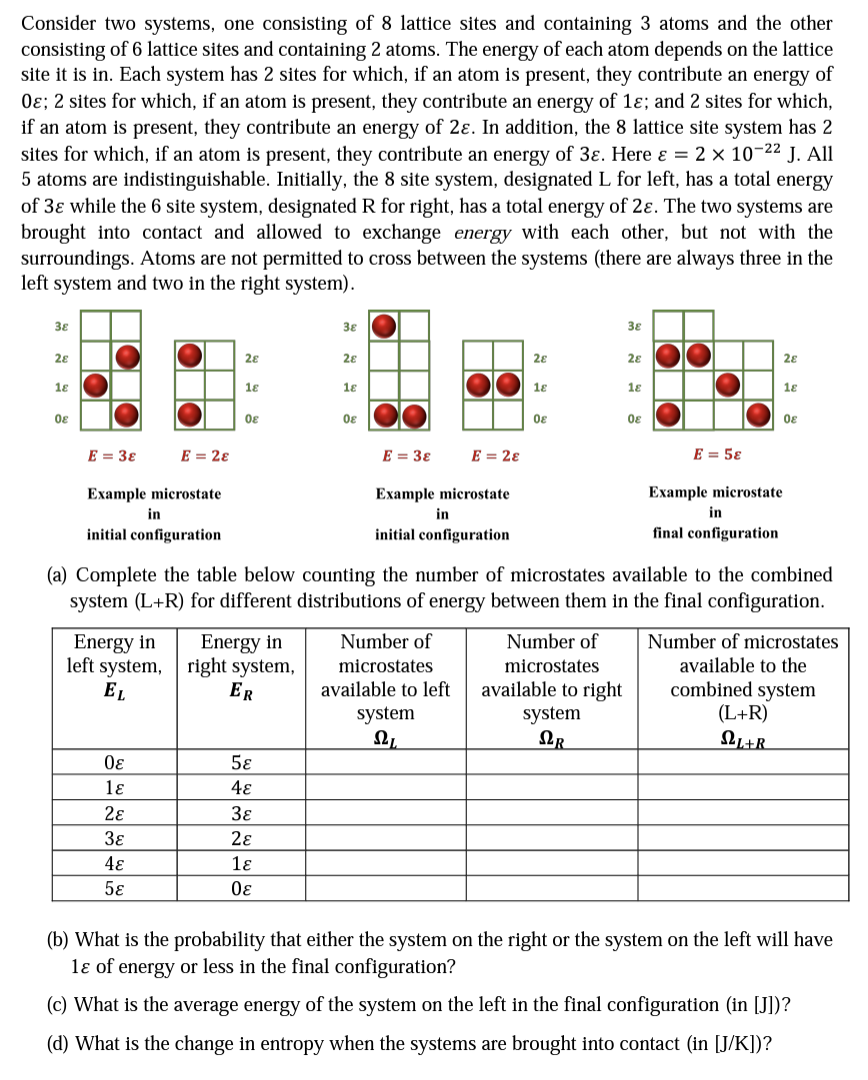
Consider two systems, one consisting of 8 lattice sites and containing 3 atoms and the other consisting of 6 lattice sites and containing 2 atoms. The energy of each atom depends on the lattice site it is in. Each system has 2 sites for which, if an atom is present, they contribute an energy of 0; 2 sites for which, if an atom is present, they contribute an energy of 1; and 2 sites for which, if an atom is present, they contribute an energy of 2. In addition, the 8 lattice site system has 2 sites for which, if an atom is present, they contribute an energy of 3. Here & = 2 10- J. All 5 atoms are indistinguishable. Initially, the 8 site system, designated L for left, has a total energy of 3 while the 6 site system, designated R for right, has a total energy of 2. The two systems are brought into contact and allowed to exchange energy with each other, but not with the surroundings. Atoms are not permitted to cross between the systems (there are always three in the left system and two in the right system). 38 28 18 08 E = 38 Example microstate in initial configuration Energy in left system, EL E = 28 0 1 2 3 4 5 28 18 08 Energy in right system, ER 38 5 4 3 2 1 0 28 18 0 E = 38 E = 28 Example microstate in initial configuration 28 Number of microstates available to left system , 18 08 38 Number of microstates available to right system QR 28 18 (a) Complete the table below counting the number of microstates available to the combined system (L+R) for different distributions of energy between them in the final configuration. 08 E = 58 28 18 0 Example microstate in final configuration Number of microstates available to the combined system (L+R) L+R (b) What is the probability that either the system on the right or the system on the left will have 1 of energy or less in the final configuration? (c) What is the average energy of the system on the left in the final configuration (in [J])? (d) What is the change in entropy when the systems are brought into contact (in [J/K])?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


