Question
Calculate the enthalpy of formation of methanol (CH3OH) from its elements, using the heats of combustion below: C (graphite) + 2 H2 (g) +
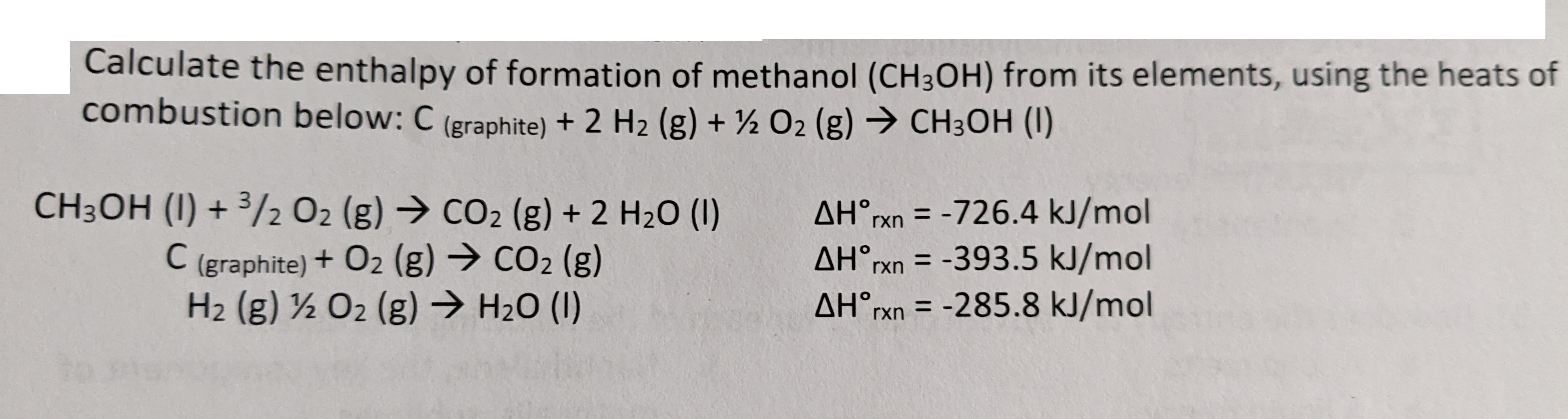
Calculate the enthalpy of formation of methanol (CH3OH) from its elements, using the heats of combustion below: C (graphite) + 2 H2 (g) + O2 (g) CH3OH (1) CH3OH (1) + 3/2 O2 (g) CO2 (g) + 2 H2O (1) C (graphite) + O2 (g) CO2 (g) H2 (g) 12 O2 (g) HO (1) AHrxn=-726.4 kJ/mol AHrxn=-393.5 kJ/mol AHrxn=-285.8 kJ/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Personal Finance
Authors: Thomas Garman, Raymond Forgue
12th edition
9781305176409, 1133595839, 1305176405, 978-1133595830
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



