Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Considering the groundwater sample .4 having a pH of 7; temperature is 25C and the concentration of HCO3 is 20 mg/L assuming that a =
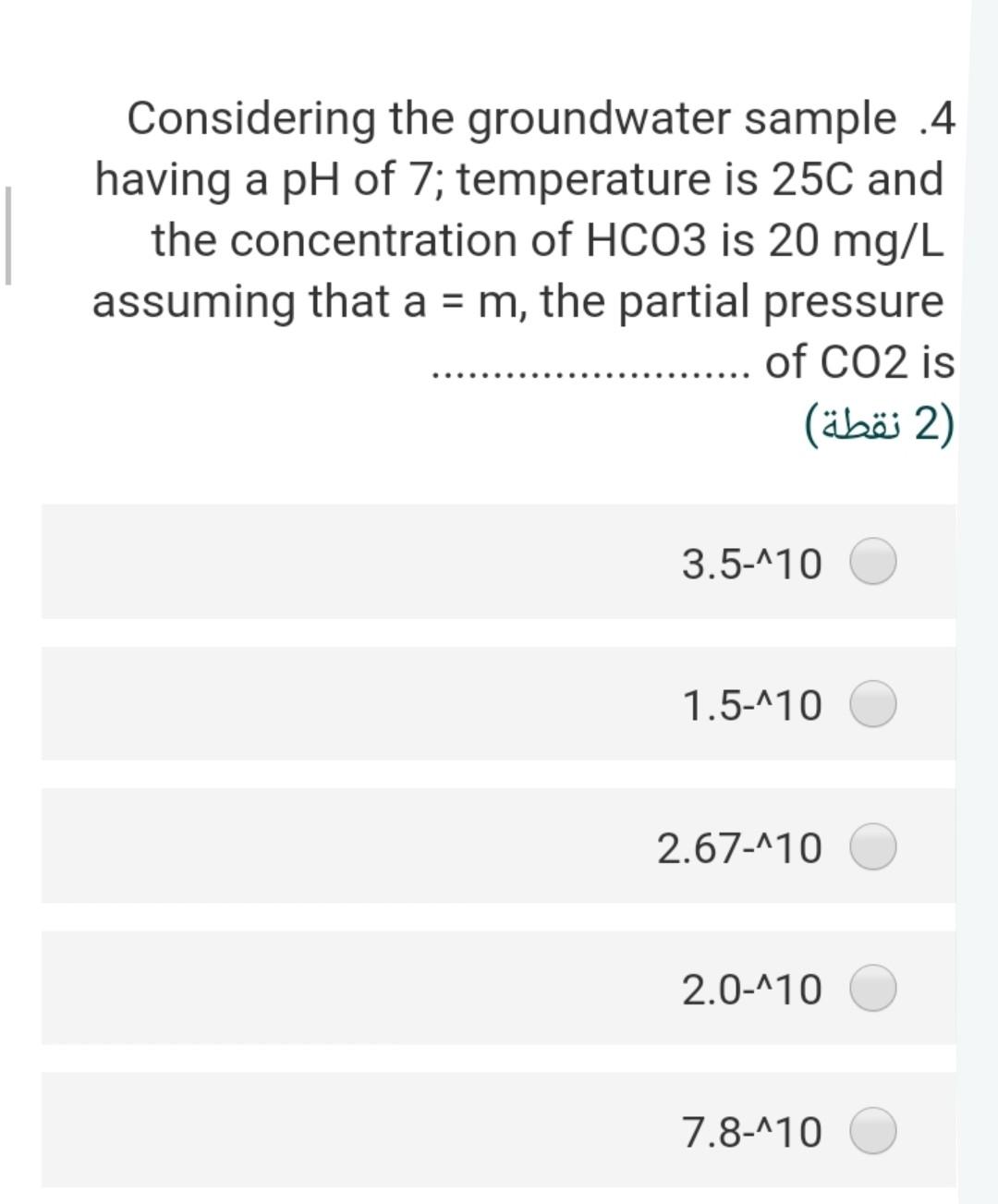
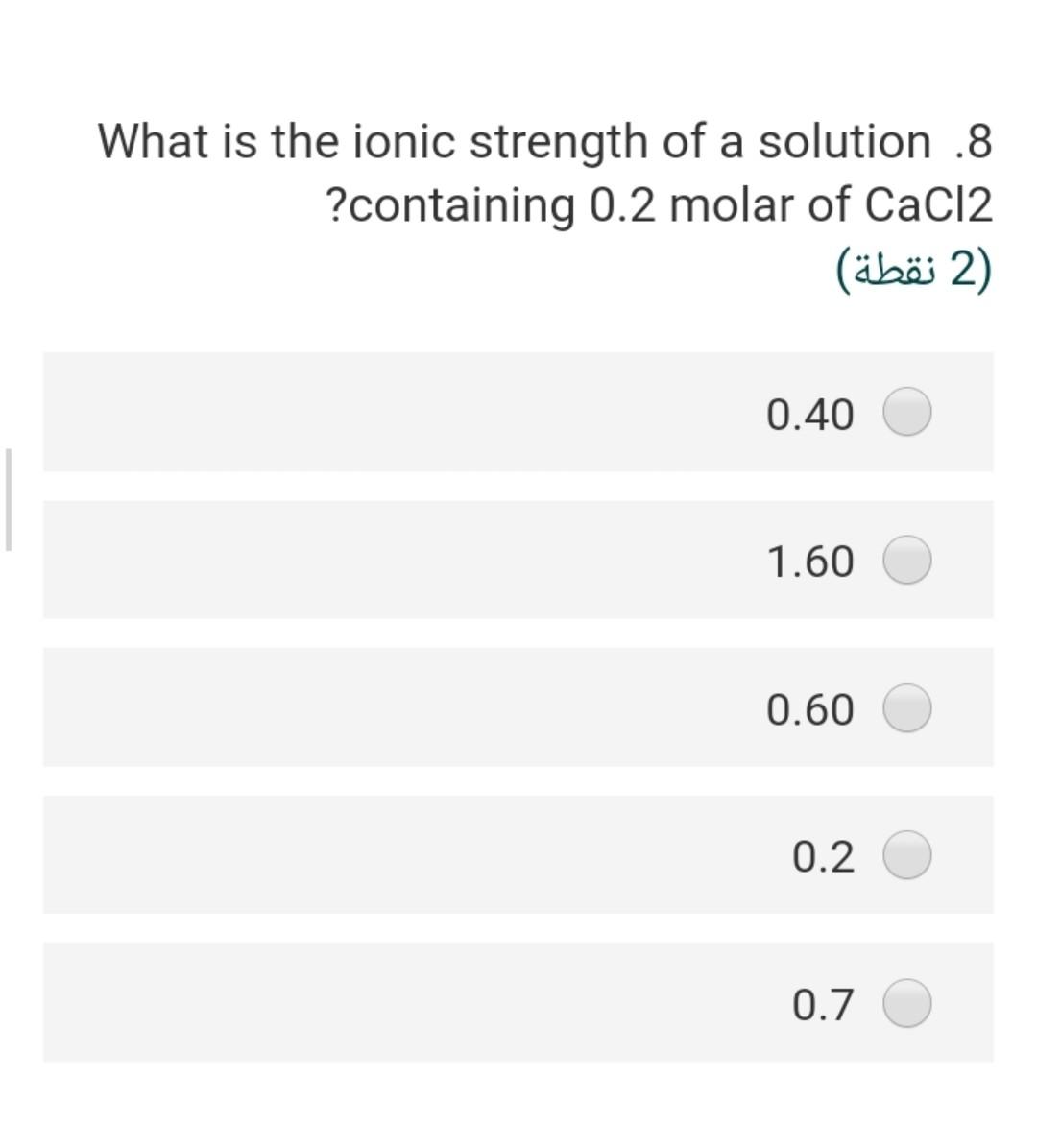


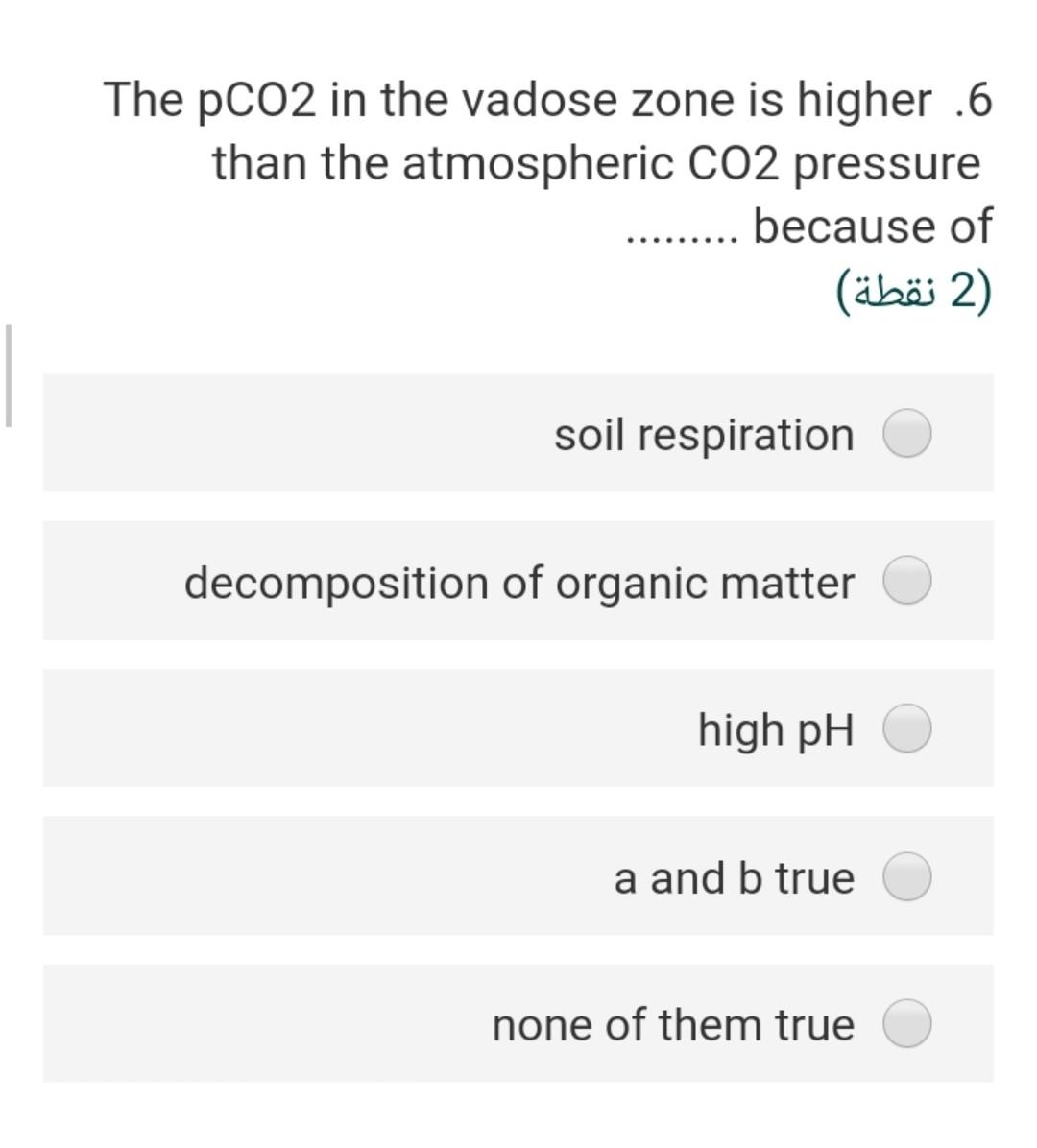
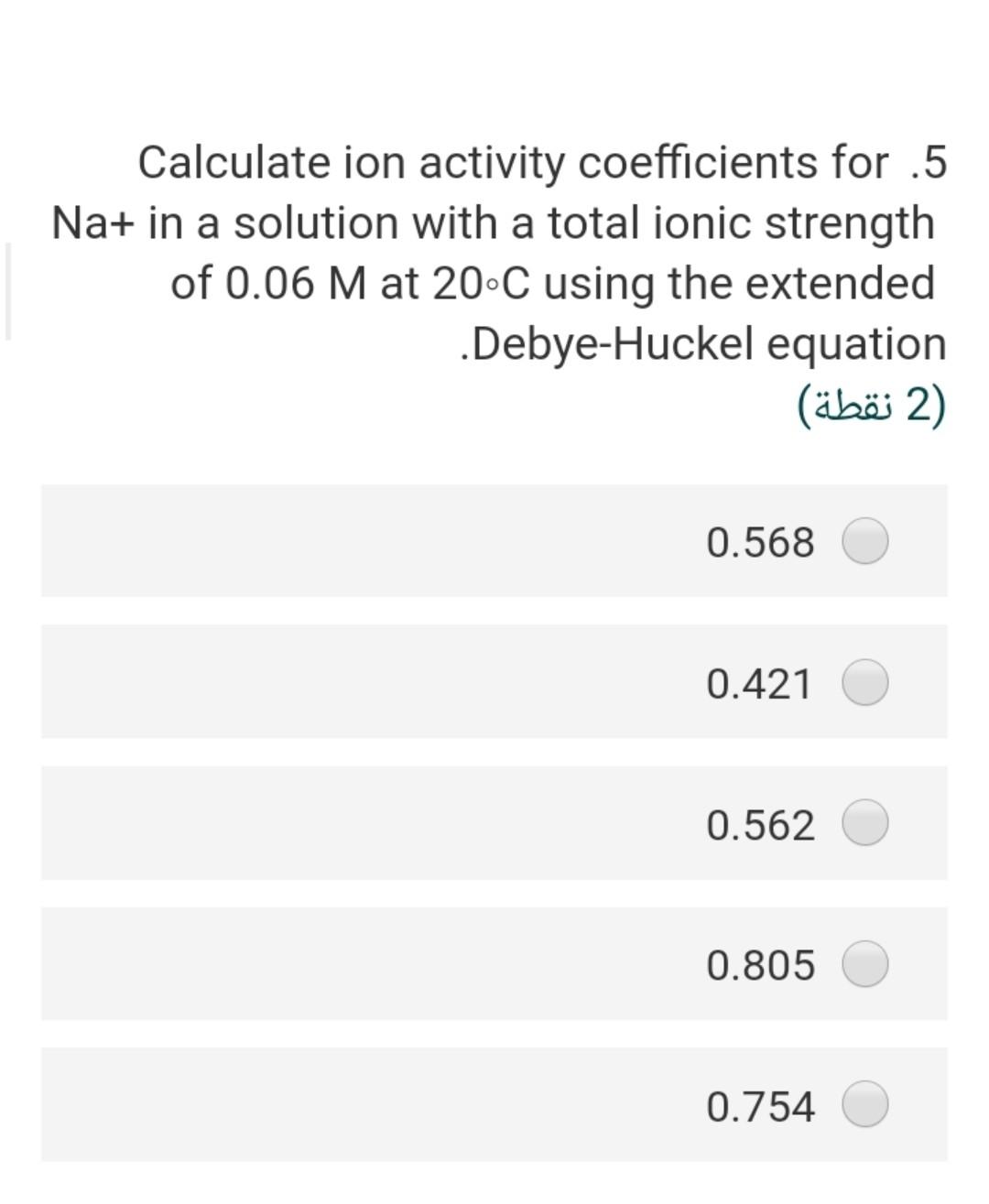

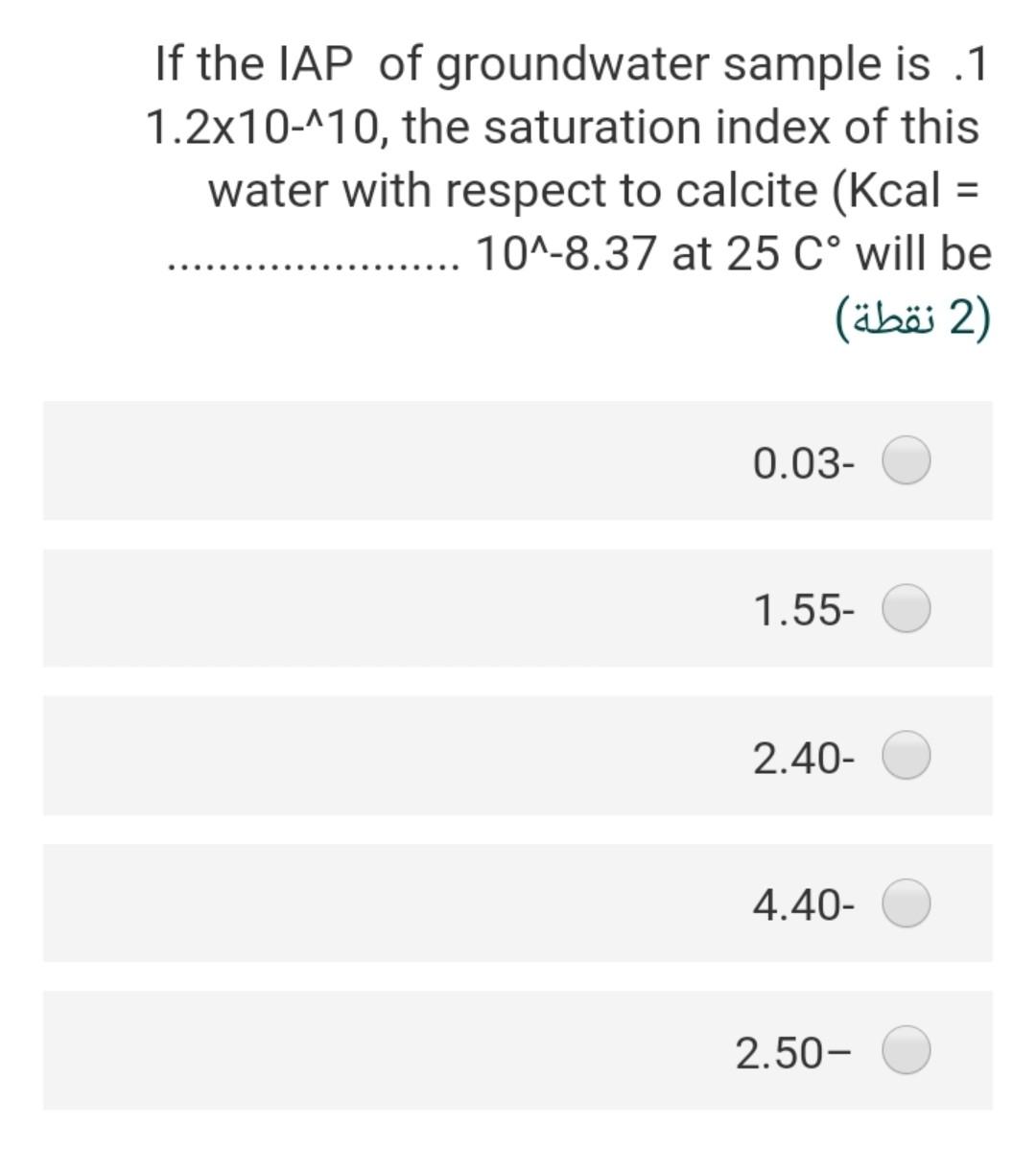


Considering the groundwater sample .4 having a pH of 7; temperature is 25C and the concentration of HCO3 is 20 mg/L assuming that a = m, the partial pressure of CO2 is 2) (2 ) 3.5-410 1.5-110 2.67-410 2.0-110 7.8-110 What is the ionic strength of a solution .8 ?containing 0.2 molar of CaCl2 2) (2 ) 0.40 1.60 0.60 0.2 0.7 The solubility product of the gypsum .17 mineral at 25 C, if the AGr =+24.2 kJ/mol will be 2 (2 ) 8.24-10 4.241-10 3.5^-10 2.31-10 17-110 If the IAP of groundwater sample is .1 1.2x10-110, the saturation index of this water with respect to calcite (Kcal = 10^-8.37 at 25 C will be (2) 0.03- 1.55- 2.40- 4.40- 2.50- The pCo2 in the vadose zone is higher .6 than the atmospheric CO2 pressure because of (2) soil respiration decomposition of organic matter high pH a and b true none of them true Calculate ion activity coefficients for .5 Na+ in a solution with a total ionic strength of 0.06 M at 20C using the extended .Debye-Huckel equation 2 (2 ) 0.568 0.421 0.562 0.805 0.754 The concentration of H2003 (mol/l) in a .2 rainwater in equilibrium with PCO2 = 10- 11.5 equal 2) (2 ) 3-110*1 4-110*1 5-110*2 6-110* 2.6 2-110*1 If the IAP of groundwater sample is .1 1.2x10-110, the saturation index of this water with respect to calcite (Kcal = 10^-8.37 at 25 C will be (2) 0.03- 1.55- 2.40- 4.40- 2.50- The concentration of H2CO3 (mol/l) in a .2 rainwater in equilibrium with PCO2 = 10- 11.5 equal (2 (2 ) 3-110*1 4-110*1 5-110*2 6-110* 2.6 2-110*1 The stability field of water shows.9 (2 ) the region of pH and reduction potential where couples are oxidized by hydrogen ions but cannot reduce hydrogen ions the region of pH and reduction potential where couples are not oxidized by hydrogen ions but can reduce hydrogen ions the region of pH and reduction potential where couples neither are oxidized by hydrogen ions nor reduce hydrogen ions the region of pH and oxidation potential where couples neither are oxidized by hydrogen ions nor reduce hydrogen ions none of these true
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


