Answered step by step
Verified Expert Solution
Question
1 Approved Answer
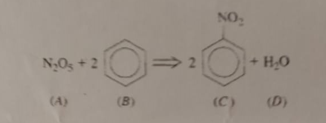
Considering the reaction below being carried out in an adiabatic CSTR reactor, calculate the volume and static time necessary to obtain 3 5 % N
Considering the reaction below being carried out in an adiabatic CSTR reactor, calculate the volume and static time necessary to obtain NO conversion? The reaction rate is first order in relation to A and second order in relation to B
exp
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


