Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Constants and information that you may need: Drag force = Cd0.5pu Af Universal gas constant, R = 8.3143 x 103 J/kmol-K. Solid propellant burning
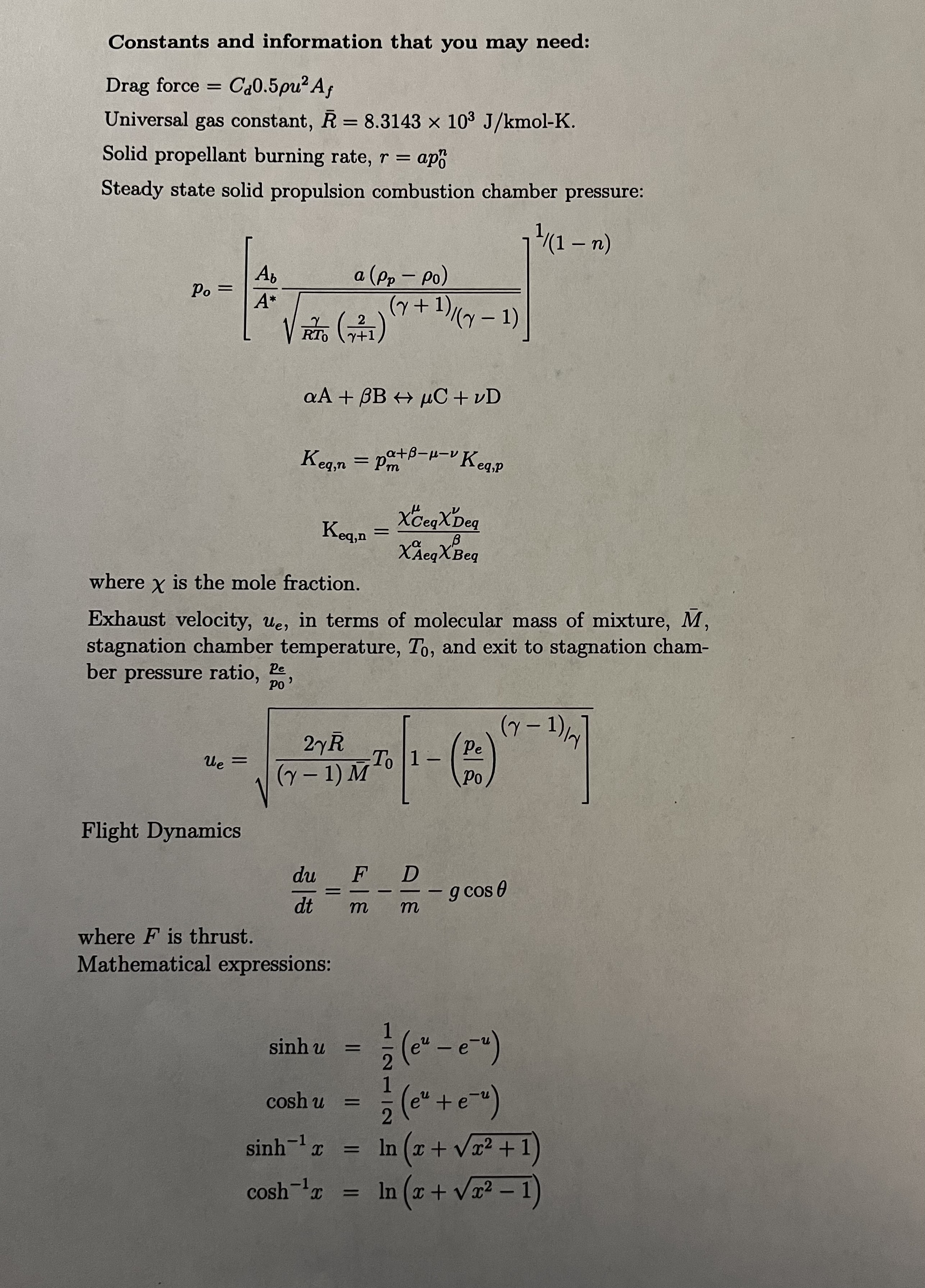
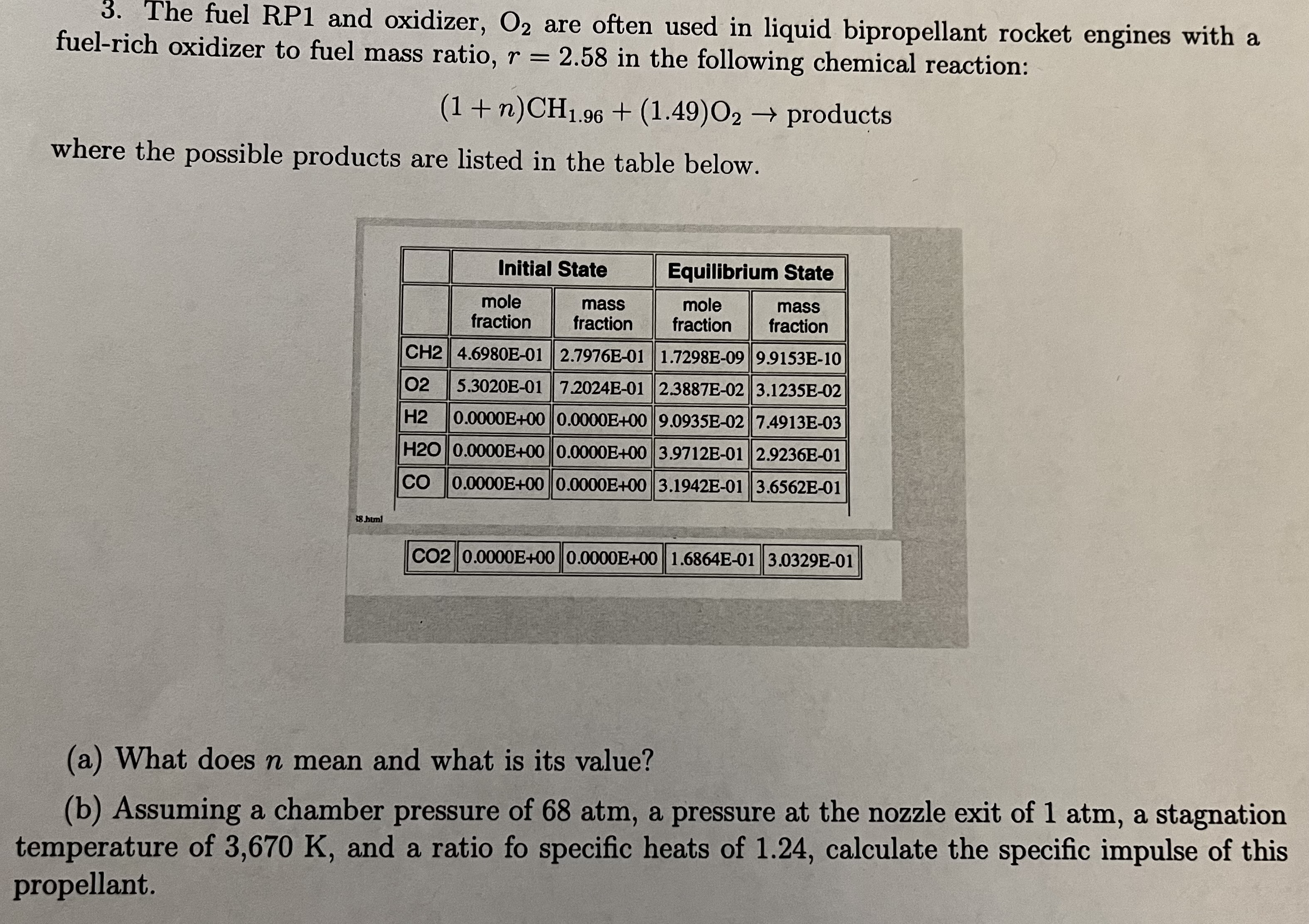
Constants and information that you may need: Drag force = Cd0.5pu Af Universal gas constant, R = 8.3143 x 103 J/kmol-K. Solid propellant burning rate, r = = ap Steady state solid propulsion combustion chamber pressure: Ab a (pp - Po) Po= A* (+ RTO (741) 2 + + 1) ( y 1) - aA+BBC+vD Keq,n = pa+B--v Keq,p XCeqXDeq Keq,n = B XAeqX Beq (1 n) where is the mole fraction. Exhaust velocity, ue, in terms of molecular mass of mixture, M, stagnation chamber temperature, To, and exit to stagnation cham- ber pressure ratio, Pe PO' ue = Flight Dynamics V (Y - 1) / Pe To 1 - 2R (Y - 1) M To du F dt m where F is thrust. Mathematical expressions: Po D m - g cos 0 sinh u = cosh u 1- (eu - e-u) 1 = 2 (e + e ) sinh-12 = ln (x+x+1) cosh 1x = In (x + x - 1) 3. The fuel RP1 and oxidizer, O2 are often used in liquid bipropellant rocket engines with a fuel-rich oxidizer to fuel mass ratio, r = 2.58 in the following chemical reaction: (1 + n)CH1.96 + (1.49)02 products where the possible products are listed in the table below. 18.html Initial State mole fraction Equilibrium State mass mole mass fraction fraction fraction CH2 4.6980E-01 2.7976E-01 1.7298E-09 9.9153E-10 02 5.3020E-01 7.2024E-01 2.3887E-02||3.1235E-02 H2 0.0000E+00 0.0000E+00 9.0935E-02 7.4913E-03 H20 0.0000E+00 0.0000E+00 3.9712E-01 2.9236E-01 CO 0.0000E+00||0.0000E+00 3.1942E-01 3.6562E-01 CO2 0.0000E+00 0.0000E+00 1.6864E-01 3.0329E-01 (a) What does n mean and what is its value? (b) Assuming a chamber pressure of 68 atm, a pressure at the nozzle exit of 1 atm, a stagnation temperature of 3,670 K, and a ratio fo specific heats of 1.24, calculate the specific impulse of this propellant.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


