Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Construct an Eh-pH diagram, at STP, for the progressive oxidation of cobalt (Co) to form oxides, starting from metallic cobalt. Indicate the stability fields of
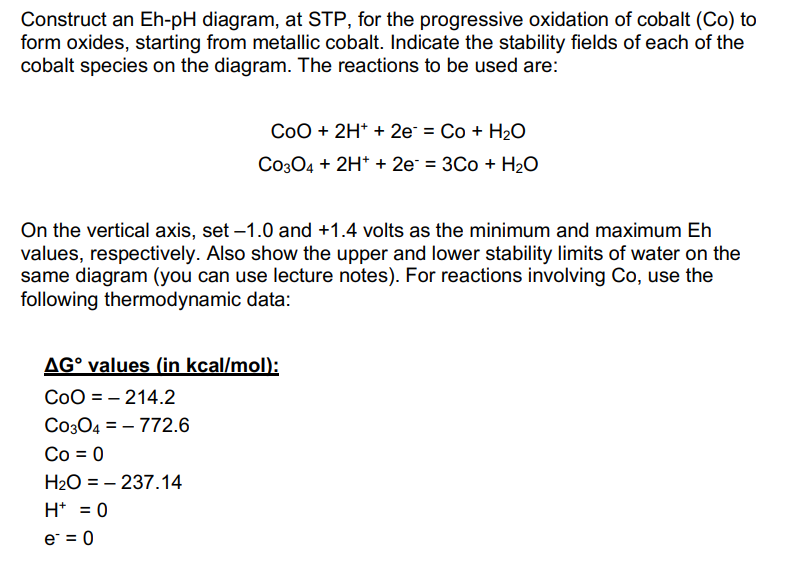 Construct an Eh-pH diagram, at STP, for the progressive oxidation of cobalt (Co) to form oxides, starting from metallic cobalt. Indicate the stability fields of each of the cobalt species on the diagram. The reactions to be used are: CoO+2H++2eCo3O4+2H++2e=Co+H2O=3Co+H2O On the vertical axis, set -1.0 and +1.4 volts as the minimum and maximum Eh values, respectively. Also show the upper and lower stability limits of water on the same diagram (you can use lecture notes). For reactions involving Co, use the following thermodynamic data: G values (in kcal/mol : CoO=214.2Co3O4=772.6Co=0H2O=237.14H+=0e=0
Construct an Eh-pH diagram, at STP, for the progressive oxidation of cobalt (Co) to form oxides, starting from metallic cobalt. Indicate the stability fields of each of the cobalt species on the diagram. The reactions to be used are: CoO+2H++2eCo3O4+2H++2e=Co+H2O=3Co+H2O On the vertical axis, set -1.0 and +1.4 volts as the minimum and maximum Eh values, respectively. Also show the upper and lower stability limits of water on the same diagram (you can use lecture notes). For reactions involving Co, use the following thermodynamic data: G values (in kcal/mol : CoO=214.2Co3O4=772.6Co=0H2O=237.14H+=0e=0 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


