Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Contraction loss coefficient, K h = Kc ] Carbon tetrachloride at ambient temperature (density = 1595 kg/m3; viscosity = 0.001 Pas) is flowing through
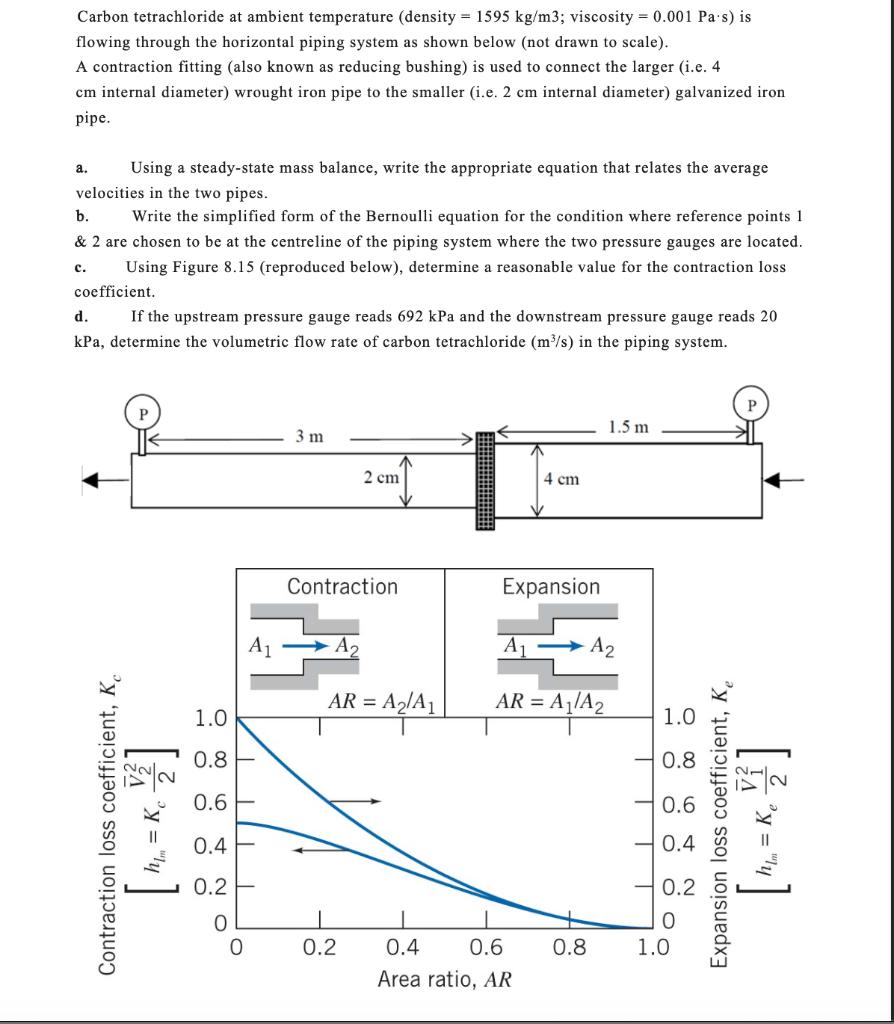
Contraction loss coefficient, K h = Kc ] Carbon tetrachloride at ambient temperature (density = 1595 kg/m3; viscosity = 0.001 Pas) is flowing through the horizontal piping system as shown below (not drawn to scale). A contraction fitting (also known as reducing bushing) is used to connect the larger (i.e. 4 cm internal diameter) wrought iron pipe to the smaller (i.e. 2 cm internal diameter) galvanized iron pipe. a. Using a steady-state mass balance, write the appropriate equation that relates the average velocities in the two pipes. b. Write the simplified form of the Bernoulli equation for the condition where reference points 1 & 2 are chosen to be at the centreline of the piping system where the two pressure gauges are located. Using Figure 8.15 (reproduced below), determine a reasonable value for the contraction loss c. coefficient. d. If the upstream pressure gauge reads 692 kPa and the downstream pressure gauge reads 20 kPa, determine the volumetric flow rate of carbon tetrachloride (m/s) in the piping system. 3 m 2 cm 4 cm 1.5 m Contraction Expansion A1 A A A2 AR = A2/A AR = A/A2 = 1.0 0.8 0.6 0.4- 0.2 1.0 T TTT 0.8 0.6 0.4 0.2 0 0 0 0.2 0.4 0.6 0.8 1.0 Area ratio, AR Expansion loss coefficient, Ke [ M = Ke V]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Based on the provided question and the information from the figure of the piping system lets address each subquestion one at a time a Using a steadystate mass balance write the appropriate equation th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


