Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determining the exact concentration of HC1. Based on the information below, what can you conclude about this lab? What is the deviation? Results: THAM
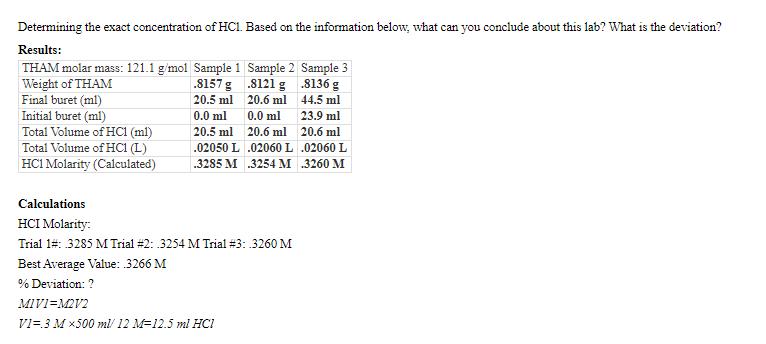
Determining the exact concentration of HC1. Based on the information below, what can you conclude about this lab? What is the deviation? Results: THAM molar mass: 121.1 g/mol Sample 1 Sample 2 Sample 3 Weight of THAM .8157 g .8121 g .8136 g Final buret (ml) 20.5 ml 20.6 ml 44.5 ml Initial buret (ml) 0.0 ml 0.0 ml 23.9 ml Total Volume of HC1 (ml) 20.5 ml 20.6 ml 20.6 ml Total Volume of HC1 (L) .02050 L .02060 L .02060 L HC1 Molarity (Calculated) .3285 M .3254 M .3260 M Calculations HCI Molarity: Trial 1#: 3285 M Trial #2: 3254 M Trial #3: .3260 M Best Average Value: .3266 M % Deviation: ? MIV1=M2V2 V1=.3 M500 ml/ 12 M-12.5 ml HCI
Step by Step Solution
★★★★★
3.26 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Answer To determine the percent deviation we first n...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


