Answered step by step
Verified Expert Solution
Question
1 Approved Answer
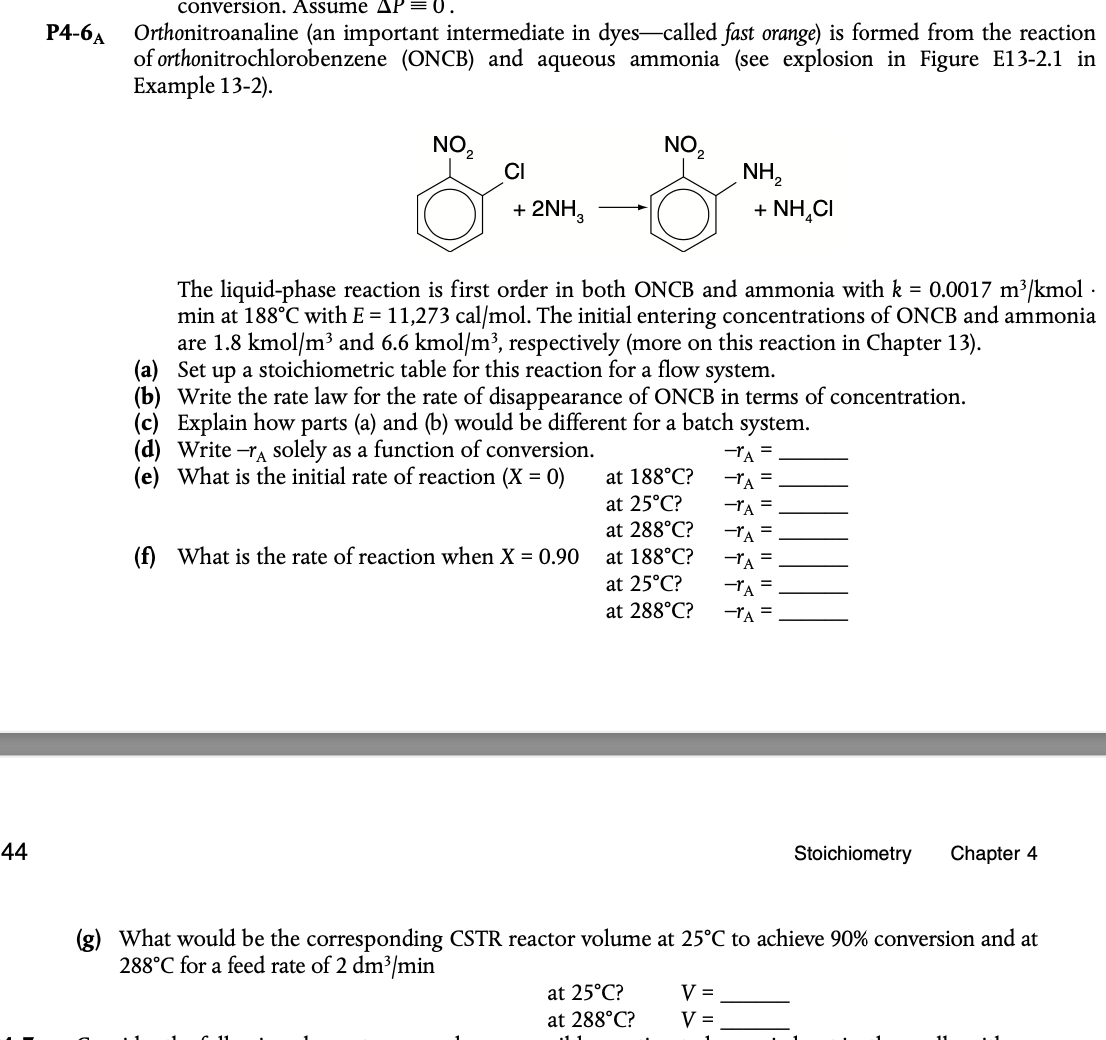
conversion. Assume P - = 0 . P 4 - 6 A Orthonitroanaline ( an important intermediate in dyes - called fast orange ) is
conversion. Assume
P A Orthonitroanaline an important intermediate in dyes called fast orange is formed from the reaction
of orthonitrochlorobenzene ONCB and aqueous ammonia see explosion in Figure E in
Example
The liquidphase reaction is first order in both ONCB and ammonia with mol.
min at with The initial entering concentrations of ONCB and ammonia
are kmo and kmo respectively more on this reaction in Chapter
a Set up a stoichiometric table for this reaction for a flow system.
b Write the rate law for the rate of disappearance of ONCB in terms of concentration.
c Explain how parts a and b would be different for a batch system.
d Write solely as a function of conversion.
e What is the initial rate of reaction at
at
at
f What is the rate of reaction when at
at
at
g What would be the corresponding CSTR reactor volume at to achieve conversion and at
for a feed rate of
at
at
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


