Question
Convert 5.85 g/L into g/100ML (note: 100 ml = 0.1L) MW = 58.5 g/mol 5.85% = 5.85%00om 5.85g 1000ml 100ml X= 0.59% What percentage
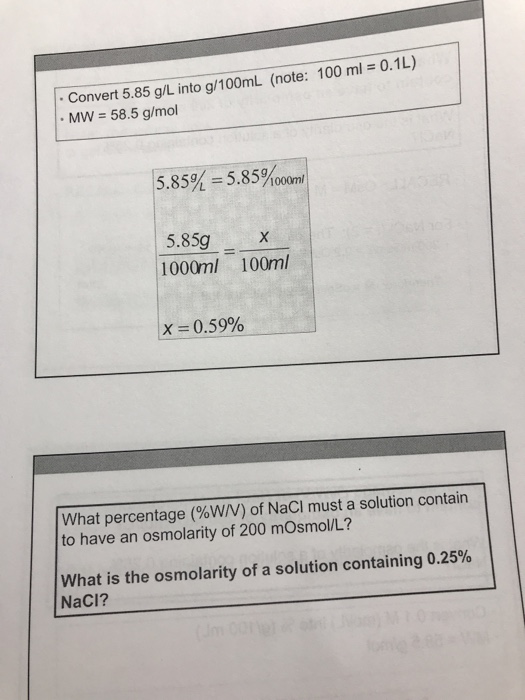
Convert 5.85 g/L into g/100ML (note: 100 ml = 0.1L) MW = 58.5 g/mol 5.85% = 5.85%00om 5.85g 1000ml 100ml X= 0.59% What percentage (%W/V) of NaCl must a solution contain to have an osmolarity of 200 mOsmol/L? What is the osmolarity of a solution containing 0.25% NaCl? (Jm 00
Step by Step Solution
3.31 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
formula 1 Osmol L W V x 10 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Analytical Chemistry
Authors: Douglas A. Skoog, Donald M. West, F. James Holler, Stanley R. Crouch
9th edition
495558281, 978-0495558286
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



