Question
Convert 94 ug/m^3 SO2 to volume mixing ratio parts per million, given that the molecular mass of SO2 is 64 g/mol. Assume the air
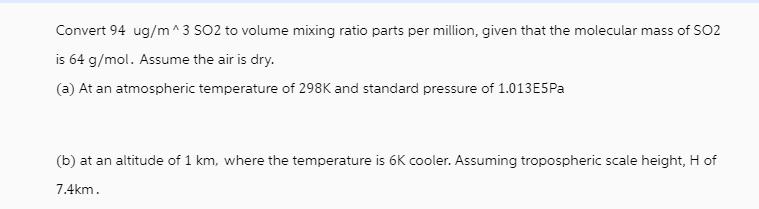
Convert 94 ug/m^3 SO2 to volume mixing ratio parts per million, given that the molecular mass of SO2 is 64 g/mol. Assume the air is dry. (a) At an atmospheric temperature of 298K and standard pressure of 1.013E5Pa (b) at an altitude of 1 km, where the temperature is 6K cooler. Assuming tropospheric scale height, H of 7.4km.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Converting SO2 concentration to volume mixing ratio Heres how to convert the SO2 concentration from ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: David Young, Shane Stadler
10th edition
1118486897, 978-1118836873, 1118836871, 978-1118899205, 1118899202, 978-1118486894
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



