Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Cooling can be realized based on waste heat using an absorption cooling device (chiller). Typically, waste heat in the form of steam or hot
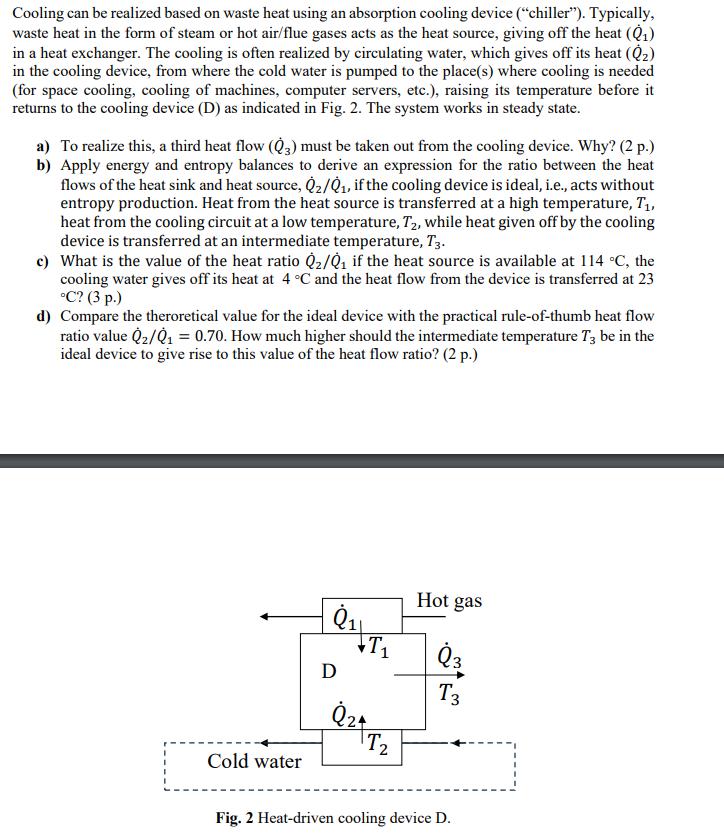
Cooling can be realized based on waste heat using an absorption cooling device ("chiller"). Typically, waste heat in the form of steam or hot air/flue gases acts as the heat source, giving off the heat () in a heat exchanger. The cooling is often realized by circulating water, which gives off its heat () in the cooling device, from where the cold water is pumped to the place(s) where cooling is needed (for space cooling, cooling of machines, computer servers, etc.), raising its temperature before it returns to the cooling device (D) as indicated in Fig. 2. The system works in steady state. a) To realize this, a third heat flow (3) must be taken out from the cooling device. Why? (2 p.) b) Apply energy and entropy balances to derive an expression for the ratio between the heat flows of the heat sink and heat source, Q/, if the cooling device is ideal, i.e., acts without entropy production. Heat from the heat source is transferred at a high temperature, T, heat from the cooling circuit at a low temperature, T, while heat given off by the cooling device is transferred at an intermediate temperature, T3. c) What is the value of the heat ratio Q/0 if the heat source is available at 114 C, the cooling water gives off its heat at 4 C and the heat flow from the device is transferred at 23 C? (3 p.) d) Compare the theroretical value for the ideal device with the practical rule-of-thumb heat flow ratio value Q/Q = 0.70. How much higher should the intermediate temperature T3 be in the ideal device to give rise to this value of the heat flow ratio? (2 p.) Cold water 81 D T Q24 T Hot gas Q3 T3 Fig. 2 Heat-driven cooling device D.
Step by Step Solution
★★★★★
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION a A third heat flow Q3 must be taken out from the cooling device because the system must be in steadystate equilibrium meaning that the rate of heat input from the heat source must be equal t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


