Question
Copper(I) ions in aqueous solution react with NH, (aq) according to Cu*(aq) + 2 NH,(aq) Cu(NH, )* (aq) Kf = 6.3 x 1010 Calculate
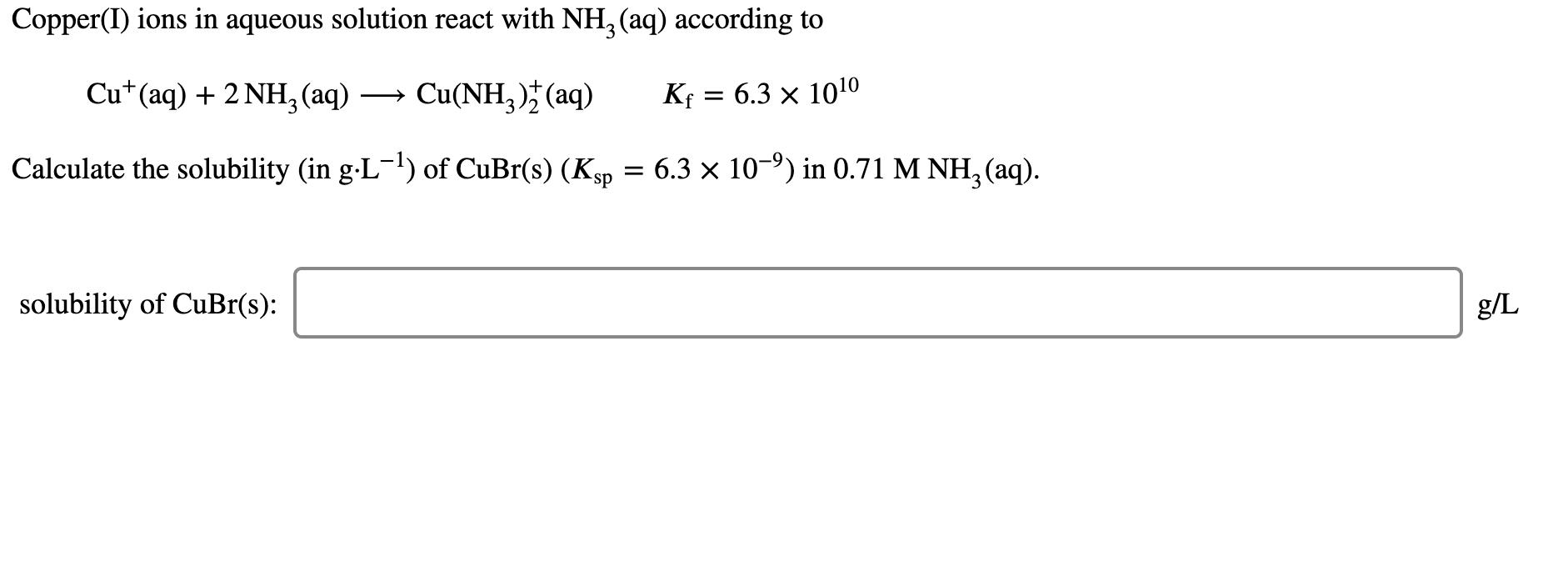
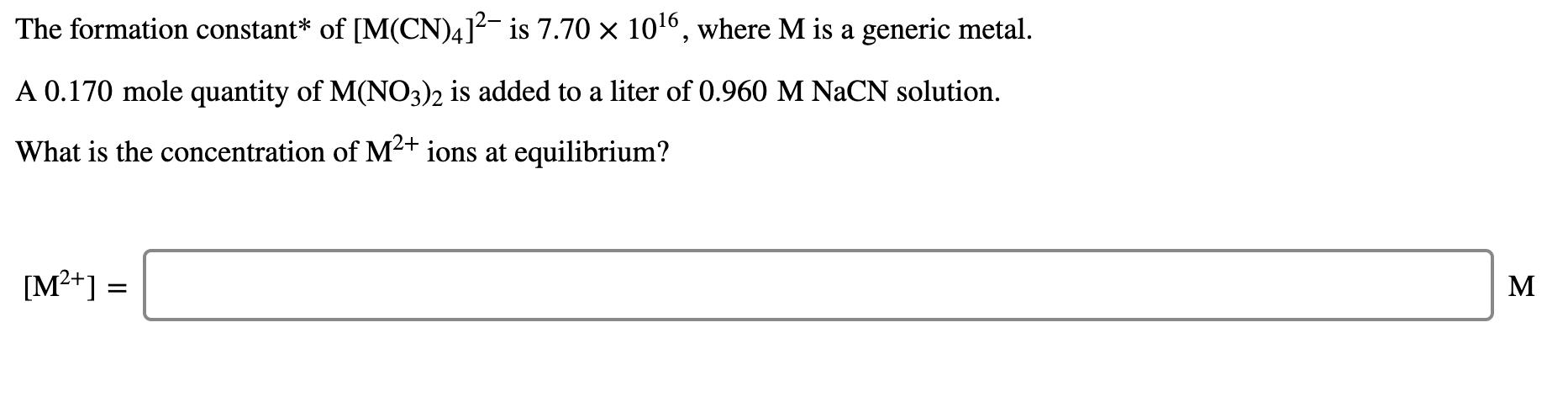
Copper(I) ions in aqueous solution react with NH, (aq) according to Cu*(aq) + 2 NH,(aq) Cu(NH, )* (aq) Kf = 6.3 x 1010 Calculate the solubility (in g-L') of CuBr(s) (Ksp = 6.3 x 10-9) in 0.71 M NH, (aq). solubility of CuBr(s): g/L The formation constant* of [M(CN)4]?- is 7.70 1016, where M is a generic metal. A 0.170 mole quantity of M(N03)2 is added to a liter of 0.960 M NACN solution. What is the concentration of M+ ions at equilibrium? [M+] = M
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



