Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Correct and Step by step explanation is needed here and solve by using Graph paper... explained properly. ., 200kg of an aqueous solution containing 15%
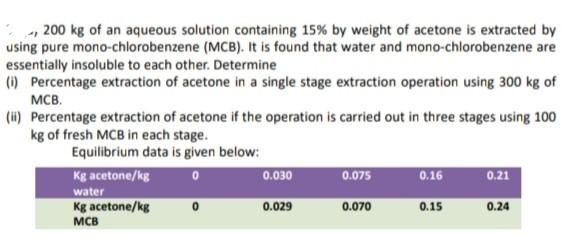
Correct and Step by step explanation is needed here and solve by using Graph paper... explained properly.
., 200kg of an aqueous solution containing 15% by weight of acetone is extracted by using pure mono-chlorobenzene (MCB). It is found that water and mono-chlorobenzene are essentially insoluble to each other. Determine (i) Percentage extraction of acetone in a single stage extraction operation using 300kg of MCB. (ii) Percentage extraction of acetone if the operation is carried out in three stages using 100 kg of fresh MCB in each stage. Equilibrium data is given belowStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


