Answered step by step
Verified Expert Solution
Question
1 Approved Answer
... Correct answers: pseudo first-order reaction pseudo 1st order reaction pseudo 1st-order reaction pseudo first order reaction 77, Fill in the blank: For irreversible SECOND-order
...
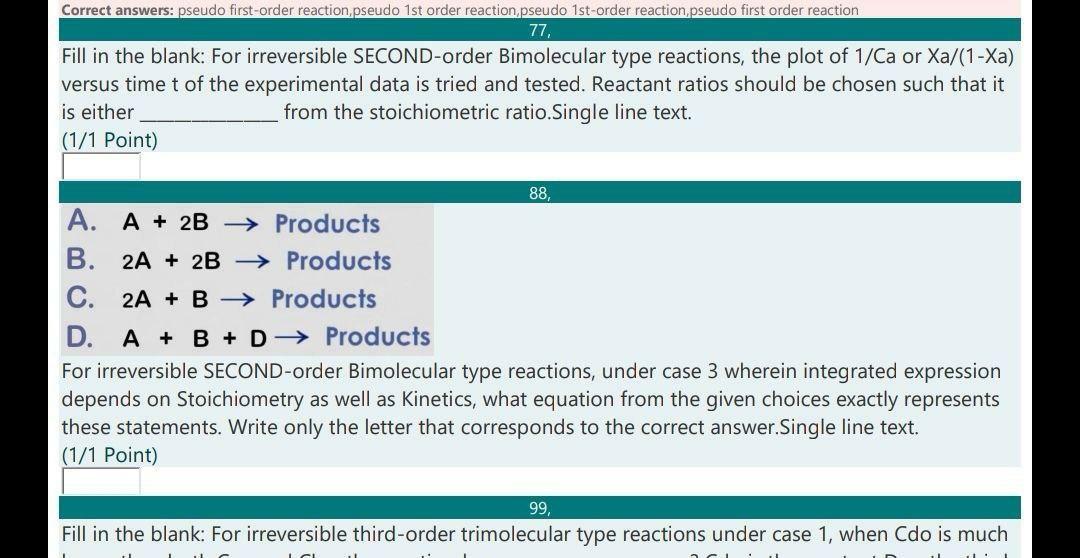
Correct answers: pseudo first-order reaction pseudo 1st order reaction pseudo 1st-order reaction pseudo first order reaction 77, Fill in the blank: For irreversible SECOND-order Bimolecular type reactions, the plot of 1/Ca or Xa/(1-Xa) versus time t of the experimental data is tried and tested. Reactant ratios should be chosen such that it is either from the stoichiometric ratio.Single line text. (1/1 Point) 88, A. A + 2B Products B. 2A + 2B Products C. 2A + B Products D. A + B + D Products For irreversible SECOND-order Bimolecular type reactions, under case 3 wherein integrated expression depends on Stoichiometry as well as Kinetics, what equation from the given choices exactly represents these statements. Write only the letter that corresponds to the correct answer.Single line text. (1/1 Point) 99, Fill in the blank: For irreversible third-order trimolecular type reactions under case 1, when Cdo is much
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


