Question
Corrosion of iron in contact with water. One region of the iron acts as the cathode and another region acts as the anode. Fe
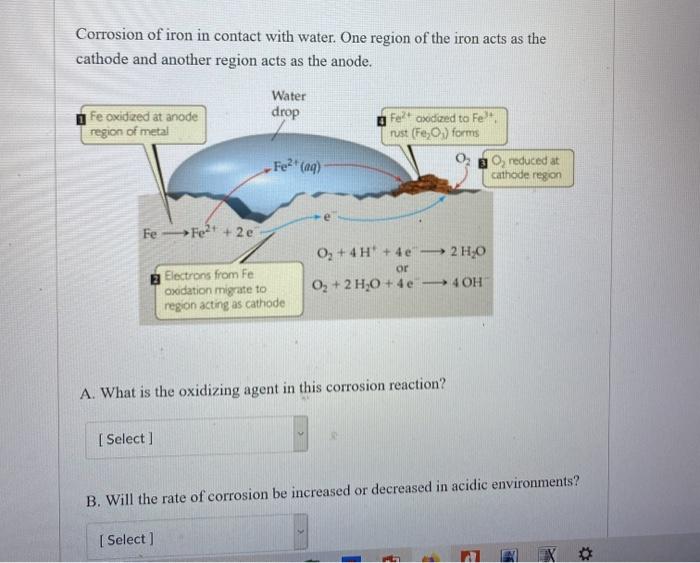
Corrosion of iron in contact with water. One region of the iron acts as the cathode and another region acts as the anode. Fe oxidized at anode region of metal Fe-Fe+ + 2e Water drop [Select] Fe+ (aq) Electrons from Fe oxidation migrate to region acting as cathode e Fe+ axidized to Fe+, rust (Fe,O) forms O + 4H + 4e 2 HO or O + 2 HO +4e -40H A. What is the oxidizing agent in this corrosion reaction? O, reduced at cathode region B. Will the rate of corrosion be increased or decreased in acidic environments? [ Select] *
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Ans A 02 is the oxidising agent in this corrosion rea...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



