Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Could you please help me with these questions Determining the Boiling Point Elevation Constant of Water 1. Pour approximately 25mL of distilled water into a
Could you please help me with these questions 
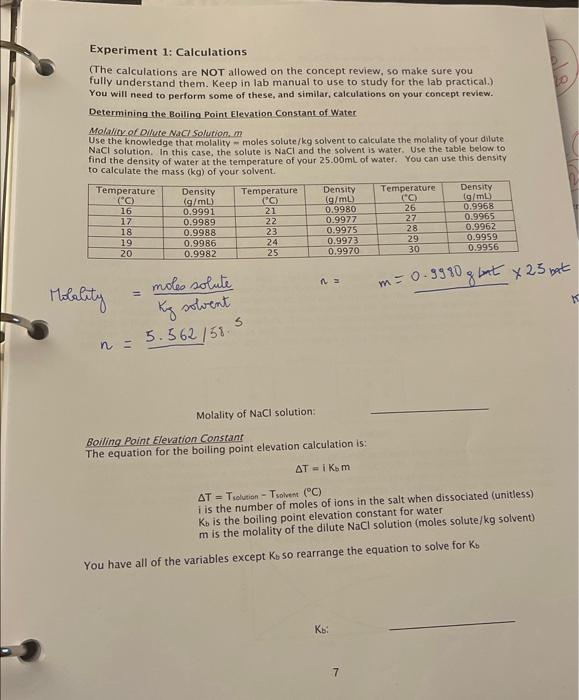

Determining the Boiling Point Elevation Constant of Water 1. Pour approximately 25mL of distilled water into a 50mL beaker. 2. Place the beaker of water on the hot plate and gradually heat the water until it is boiling. vigorously. The temperature should reach a constant value. 3. Insert your temperature probe into the boiling water. Wait until a steady temperature is reached. 4. Record this temperature as Tiovers. 5. While the beaker of pure water is being heated, record the mass of a second 50mL beaker. 6. Remove the beaker from the balance. 7. Prepare a dilute NaCl solution by measuring out 5-6g of NaCl into the pre-weighed 50mL 8. Record the exact mass of the beaker and salt. 36.722g Mass of beaker and salt: 9. Use a graduated cylinder to measure 25.00mL distilled water. Record the temperature of this water. You will use this temperature to determine the density of water, which will be needed to determine the mass of water to use in your calculation of molality. Temperature of the 25.00mL of water: 21.40 10. Add the 25.00mL of water to the 50mL beaker containing the salt. 11. Mix well. 12. Once you have recorded the temperature of the pure boiling water (step 4), remove the beaker of pure water and place the beaker of dilute NaCl solution on the hot plate. 13. Slowly heat the NaCl solution. 14. When the temperature reaches approximately 90C, watch the solution carefully until you see bubbles in the solution. Carefully monitor the solution until it comes to a full boil. 15. Immediately record the temperature of the boiling solution. Tsolution: 1040 16. Discard the NaCl solution into the waste container. Experiment 1: Calculations The calculations are NOT allowed on the concept review, so make sure you fully understand them. Keep in lab manual to use to study for the lab practical.) You will need to perform some of these, and similar, calculations on your concept review. Determining the Boiling Point Elevation Constant of Water Molalite of Ollute NaCl solution, m. Use the knowledge that molality - moles solute /kg solvent to calculate the molality of your dilute NaCl solution. In this case, the solute is NaCl and the solvent is water. Use the table below to find the density of water at the temperature of your 25.00mL of water. You can use this density to calculate the mass (kg) of your solvent. alityn=Kysotventmoleosolute=5.562/58.5 Molality of NaCl solution: Boiling Point Elevation Constant The equation for the boiling point elevation calculation is: T=iKbm T=TsolvianTsolven(C) i is the number of moles of ions in the salt when dissociated (unitless Kbb is the boiling point elevation constant for water m is the molality of the dilute NaCl solution (moles solute/kg solvent You have all of the variables except Kb so rearrange the equation to solve for Kb Bercent Exar in K The accepted value for Ki of water is 0.52C/m. Calculate the percent ermo in your value for K5 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


