Answered step by step
Verified Expert Solution
Question
1 Approved Answer
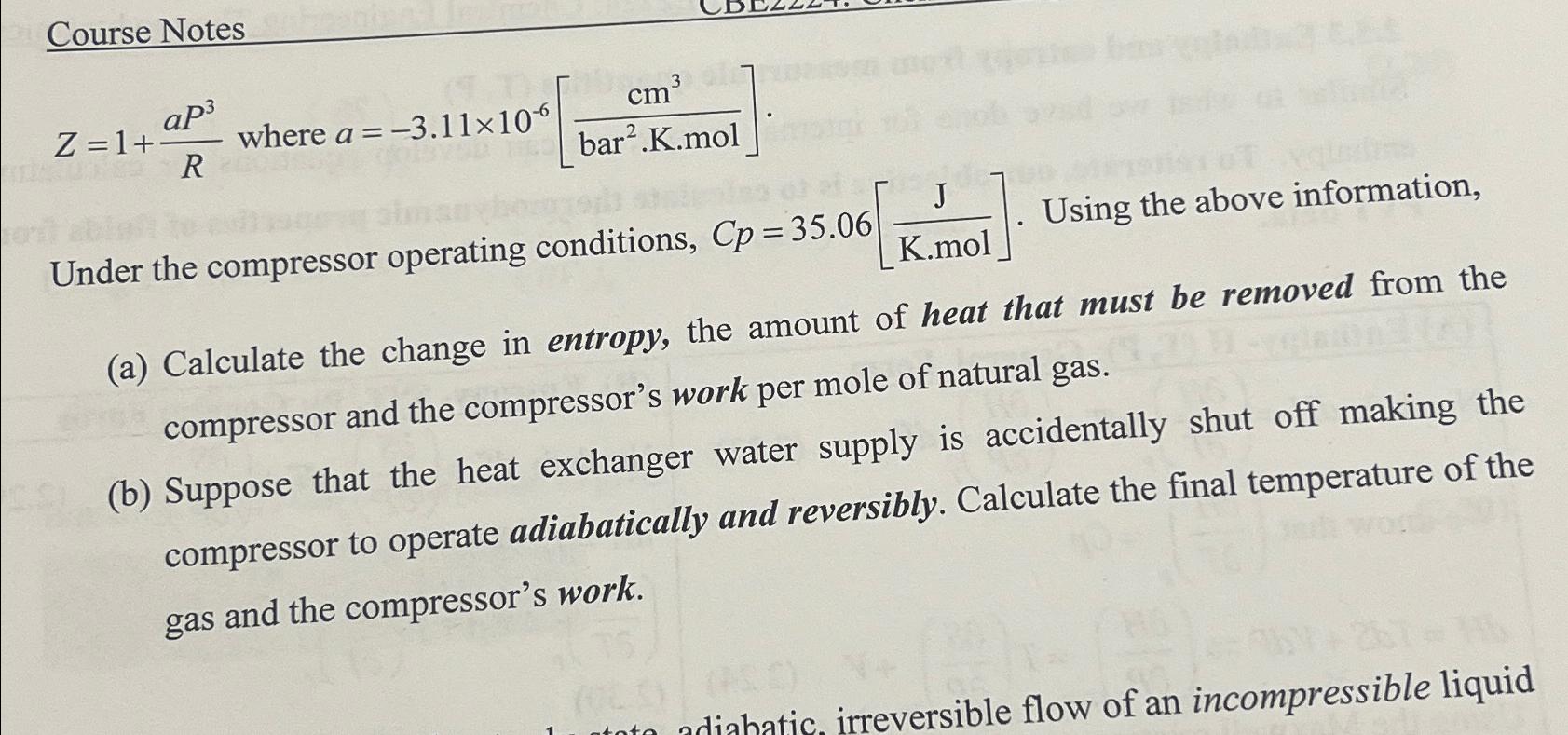
Course Notes Z=1+(aP^(3))/(R) where a=-3.11times 10^(-6)[(cm^(3))/((/bar ^(( ) 2)*K*mol)]. Under the compressor operating conditions, Cp=35.06[(J)/(K*mol)] . Using the above information, (a) Calculate the change
Course Notes\
Z=1+(aP^(3))/(R) where a=-3.11\\\\times 10^(-6)[(cm^(3))/((/bar ^(()
2)*K*mol)].\ Under the compressor operating conditions,
Cp=35.06[(J)/(K*mol)]. Using the above information,\ (a) Calculate the change in entropy, the amount of heat that must be removed from the compressor and the compressor's work per mole of natural gas.\ (b) Suppose that the heat exchanger water supply is accidentally shut off making the compressor to operate adiabatically and reversibly. Calculate the final temperature of the gas and the compressor's work.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


