Answered step by step
Verified Expert Solution
Question
1 Approved Answer
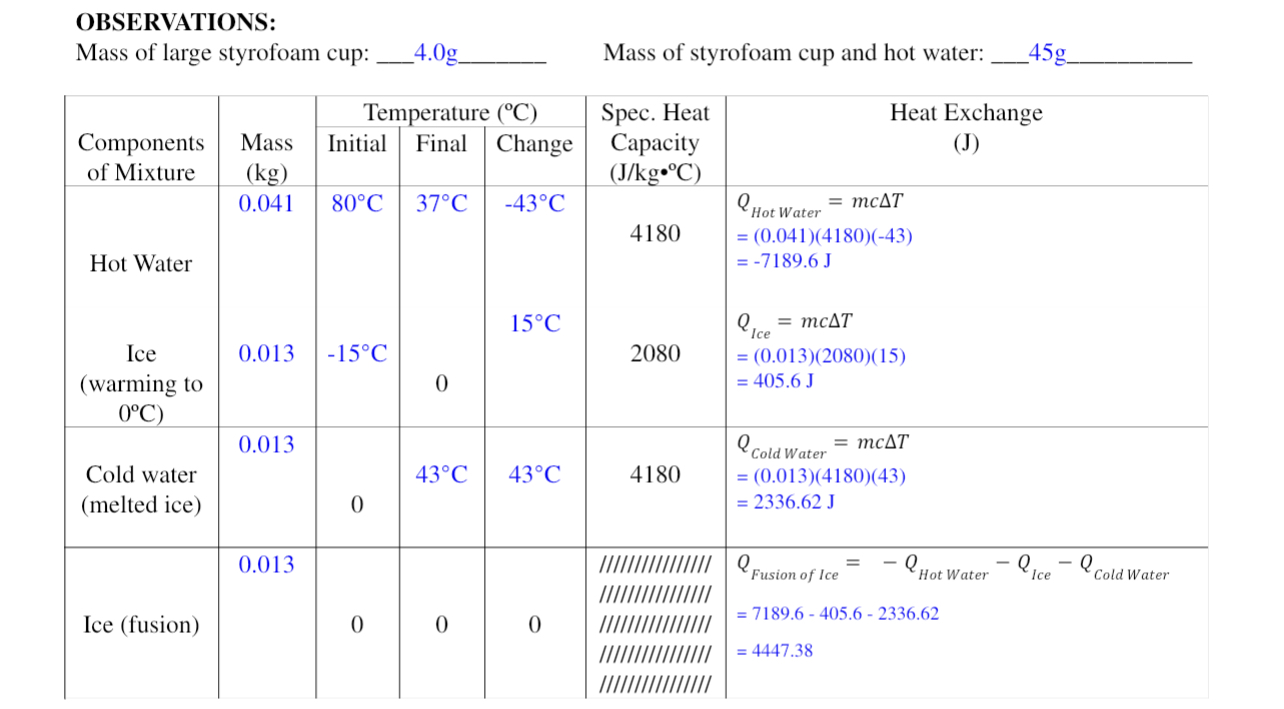
Create a Temperature vs Time graph using the attached data set below with two lines, one for the Ice/Cold Water, and another for the Hot
Create a Temperature vs Time graph using the attached data set below with two lines, one for the Ice/Cold Water, and another for the Hot Water. Start/end temperatures on the graph should reflect the values in the data, but time scale does not need to be indicated. For the graph, imagine that while energy is transferred between the Hot Water and the Cold Water/Ice, that they theoretically remain separate, and don't physically mix.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


