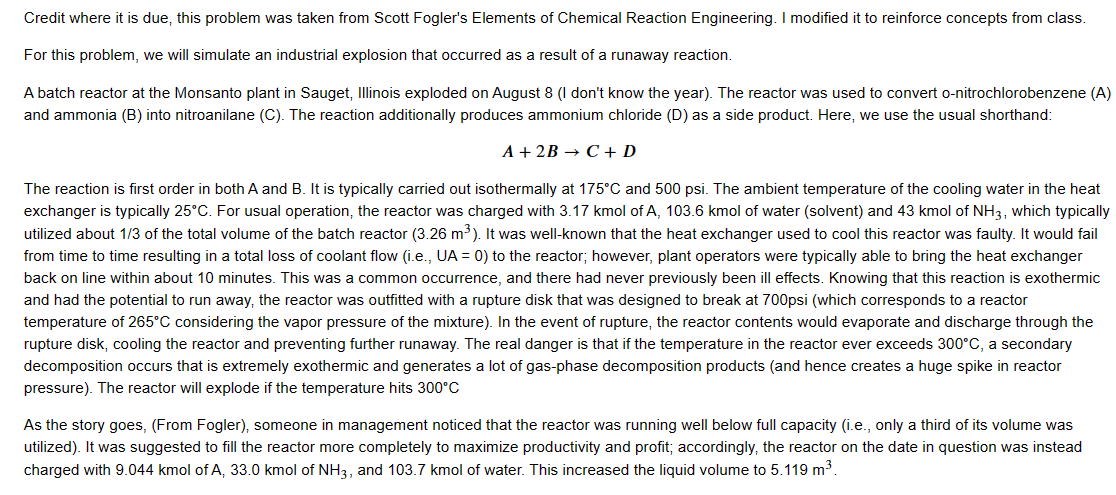
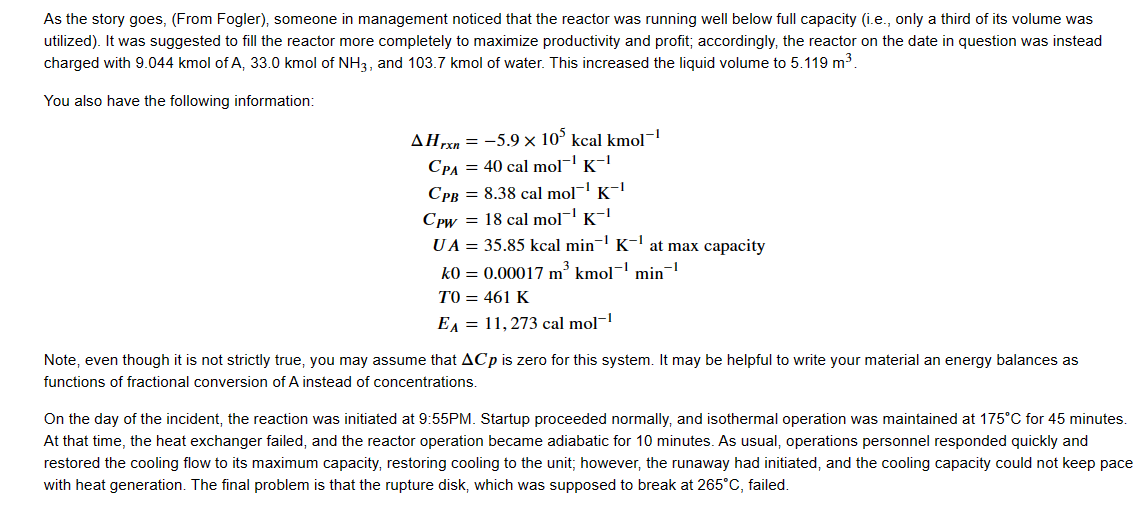


Credit where it is due, this problem was taken from Scott Fogler's Elements of Chemical Reaction Engineering. I modified it to reinforce concepts from class. For this problem, we will simulate an industrial explosion that occurred as a result of a runaway reaction. A batch reactor at the Monsanto plant in Sauget, Illinois exploded on August 8 (I don't know the year). The reactor was used to convert o-nitrochlorobenzene (A) and ammonia (B) into nitroanilane (C). The reaction additionally produces ammonium chloride (D) as a side product. Here, we use the usual shorthand: A+2BC+D The reaction is first order in both A and B. It is typically carried out isothermally at 175C and 500 psi. The ambient temperature of the cooling water in the heat exchanger is typically 25C. For usual operation, the reactor was charged with 3.17kmol of A,103.6kmol of water (solvent) and 43kmol of NH, which typically utilized about 1/3 of the total volume of the batch reactor (3.26m3). It was well-known that the heat exchanger used to cool this reactor was faulty. It would fail from time to time resulting in a total loss of coolant flow (i.e., UA=0 ) to the reactor; however, plant operators were typically able to bring the heat exchanger back on line within about 10 minutes. This was a common occurrence, and there had never previously been ill effects. Knowing that this reaction is exothermic and had the potential to run away, the reactor was outfitted with a rupture disk that was designed to break at 700 psi (which corresponds to a reactor temperature of 265C considering the vapor pressure of the mixture). In the event of rupture, the reactor contents would evaporate and discharge through the rupture disk, cooling the reactor and preventing further runaway. The real danger is that if the temperature in the reactor ever exceeds 300C, a secondary decomposition occurs that is extremely exothermic and generates a lot of gas-phase decomposition products (and hence creates a huge spike in reactor pressure). The reactor will explode if the temperature hits 300C As the story goes, (From Fogler), someone in management noticed that the reactor was running well below full capacity (i.e., only a third of its volume was utilized). It was suggested to fill the reactor more completely to maximize productivity and profit; accordingly, the reactor on the date in question was instead charged with 9.044kmol of A,33.0kmol of NH3, and 103.7kmol of water. This increased the liquid volume to 5.119m3. As the story goes, (From Fogler), someone in management noticed that the reactor was running well below full capacity (i.e., only a third of its volume was utilized). It was suggested to fill the reactor more completely to maximize productivity and profit; accordingly, the reactor on the date in question was instead charged with 9.044kmol of A,33.0kmolofNH3, and 103.7kmol of water. This increased the liquid volume to 5.119m3. You also have the following information: HrxnCPACPBCPWUAk0T0EA=5.9105kcalkmol1=40calmol1K1=8.38calmol1K1=18calmol1K1=35.85kcalmin1K1atmaxcapacity=0.00017m3kmol1min1=461K=11,273calmol1 Note, even though it is not strictly true, you may assume that Cp is zero for this system. It may be helpful to write your material an energy balances as functions of fractional conversion of A instead of concentrations. On the day of the incident, the reaction was initiated at 9:55PM. Startup proceeded normally, and isothermal operation was maintained at 175C for 45 minutes. At that time, the heat exchanger failed, and the reactor operation became adiabatic for 10 minutes. As usual, operations personnel responded quickly and restored the cooling flow to its maximum capacity, restoring cooling to the unit; however, the runaway had initiated, and the cooling capacity could not keep pace with heat generation. The final problem is that the rupture disk, which was supposed to break at 265C, failed. Starting at a time of 0 (or 9:55PM ), prepare a temperature vs. time plot for this scenario. At what time did the reactor explode










