Question
Critical thinking question (20 Points) The triple bond in alkynes can be reduced by hydrogenation reactions. Depending on the reagent used, different reduction products are
-
Critical thinking question (20 Points)
The triple bond in alkynes can be reduced by hydrogenation reactions. Depending on the reagent used, different reduction products are obtained as described below:
-
Complete reduction -Hydrogenation by H2(g) in presence of an active catalyst (Pd or Pt) in ethanol at RT, adds two moles of hydrogen across the triple bond to completely saturate the triple bond of the alkyne and forms an alkane
-
Partial stereospecific reduction - Hydrogenation by H2(g) in presence of a poisoned catalyst (Pd/BaSO4) in ethanol at RT, adds one mole of hydrogen, partially reducing the triple bond to a double bond; Where possible, a cis-alkene is produced.
-
Partial stereospecific Reduction (Birch reduction) with metallic sodium in presence of liquid ammonia, at RT, adds one mole of hydrogen, partially reducing the triple bond to a double bond; Where possible, a trans-alkene is produced. Sodium is oxidized to NaNH2.
-
The triple bond in alkynes can be oxidized using hot alkaline potassium permanganate. This oxidation leads to cleavage of the triple bond. The sp carbons of the alkyne are oxidized to carboxylic acid
Use this information to answer the following questions 25 points
Write the equations for the following reactions of the alkyne A. Indicate the reactants and product(s), reagent and reaction conditions in each case
A 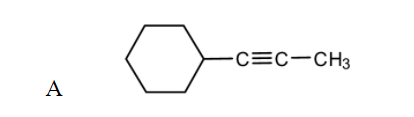
-
Partial catalytic reduction of A
-
Birch reduction of A
(c) Complete hydrogenation of A
-
Propose a synthesis for the following alkene starting from an alkyne:
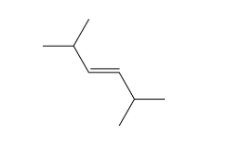
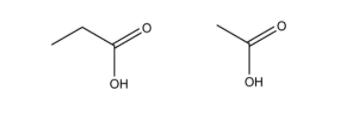
(e) An alkyne on oxidation by hot potassium permanganate produced two carboxylic acids shown below. Determine the structure of the starting alkyne
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


