Question
The boiling point of water HO is 100.0C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is sucrose. How many grams of
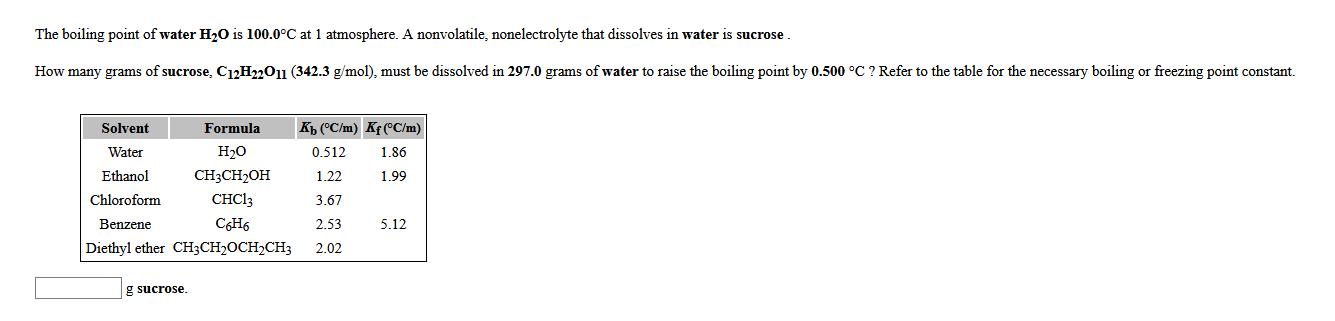
The boiling point of water HO is 100.0C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in water is sucrose. How many grams of sucrose, C12H22O11 (342.3 g/mol), must be dissolved in 297.0 grams of water to raise the boiling point by 0.500 C ? Refer to the table for the necessary boiling or freezing point constant. Formula HO CH3CHOH CHC13 C6H6 Diethyl ether CH3CHCHCH3 Solvent Water Ethanol Chloroform Benzene g sucrose. Kb (C/m) Kf (C/m) 1.86 0.512 1.22 1.99 3.67 2.53 2.02 5.12
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
w 92 918 i Vonthoff factor of Sucrose 1 Kb ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Foundations of Finance The Logic and Practice of Financial Management
Authors: Arthur J. Keown, John D. Martin, J. William Petty
8th edition
132994879, 978-0132994873
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



