Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Current Attempt in Progress ( a ) For the solidification of iron, calculate the critical radius r and the activation free energy G * *
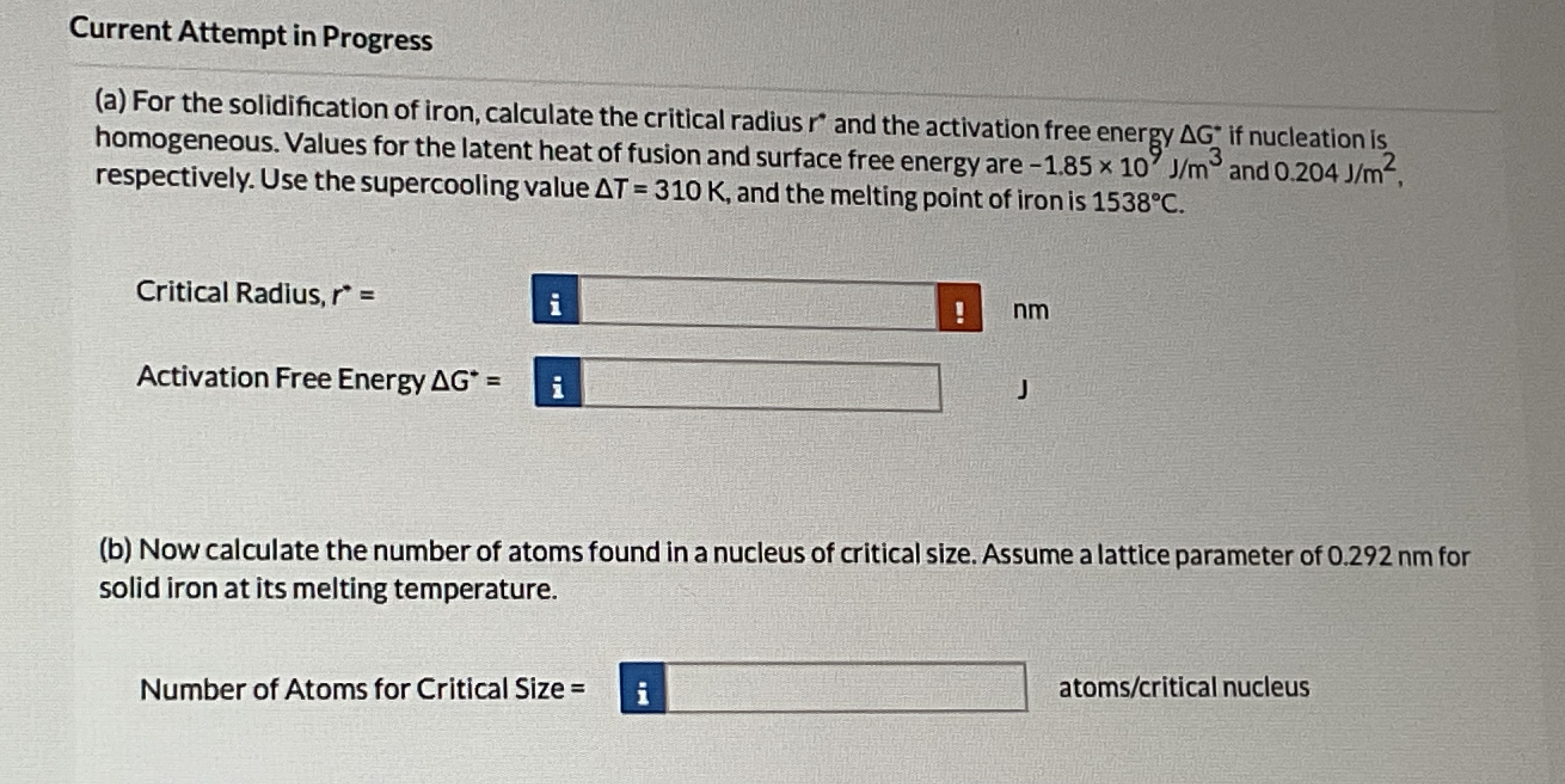
Current Attempt in Progress
a For the solidification of iron, calculate the critical radius and the activation free energy if nucleation is
homogeneous. Values for the latent heat of fusion and surface free energy are and
respectively. Use the supercooling value and the melting point of iron is
Critical Radius,
Activation Free Energy
b Now calculate the number of atoms found in a nucleus of critical size. Assume a lattice parameter of for
solid iron at its melting temperature.
Number of Atoms for Critical Size
atomscritical nucleus
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


