Question
Current Density / Acm 1. A plain steel plate 1 cm in area is in full contact with an acid solution with concentration of
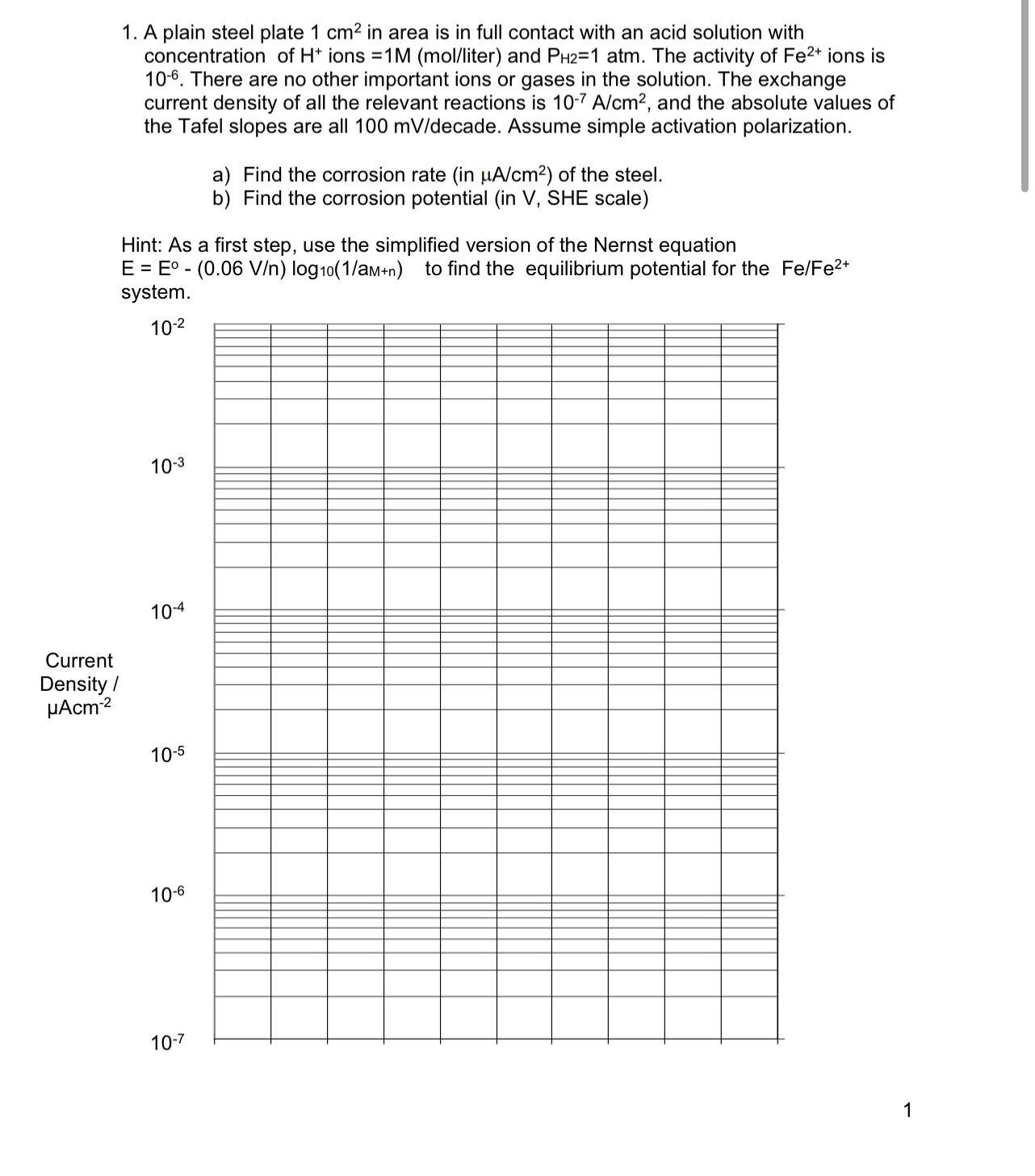
Current Density / Acm 1. A plain steel plate 1 cm in area is in full contact with an acid solution with concentration of H+ ions =1M (mol/liter) and PH2=1 atm. The activity of Fe2+ ions is 10-6. There are no other important ions or gases in the solution. The exchange current density of all the relevant reactions is 10-7 A/cm, and the absolute values of the Tafel slopes are all 100 mV/decade. Assume simple activation polarization. a) Find the corrosion rate (in A/cm) of the steel. b) Find the corrosion potential (in V, SHE scale) Hint: As a first step, use the simplified version of the Nernst equation E E (0.06 V/n) log10(1/a+n) to find the equilibrium potential for the Fe/Fe+ - system. 10-2 10-3 10-4 10-5 10-6 10-7 1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



