Question
current is applied to a molten mixture of PbF2, FeCl2, And AlBr3 Current is applied to a molten mixture of PbF2, FeCl2, and AlBr3. Standard
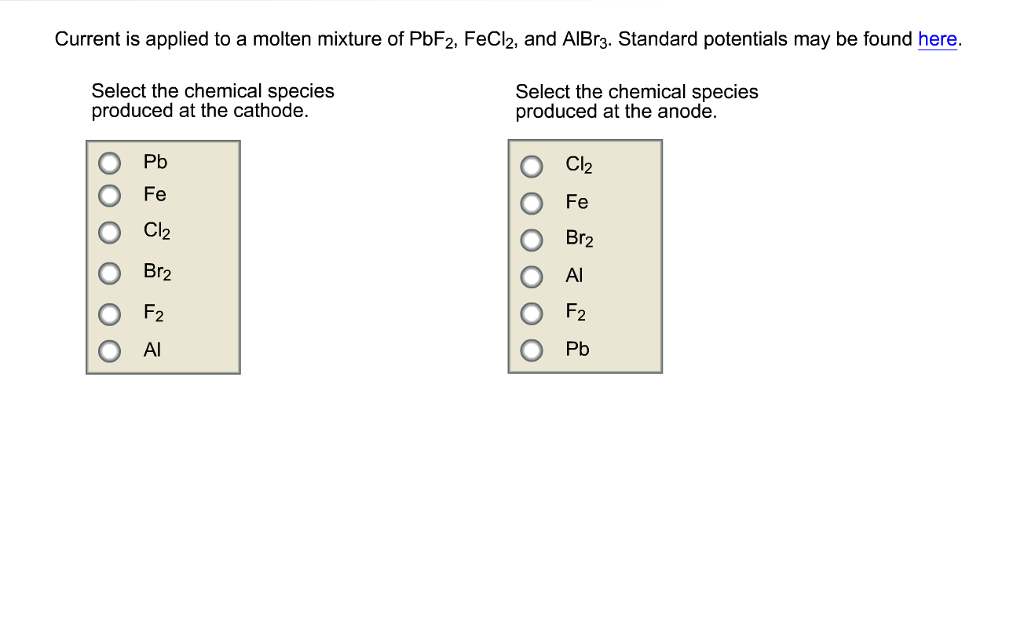
current is applied to a molten mixture of PbF2, FeCl2, And AlBr3
Current is applied to a molten mixture of PbF2, FeCl2, and AlBr3. Standard potentials may be found here. Select the chemical species produced at the cathode. Select the chemical species produced at the anode. 0.00 Pb Fe Cl Br2 F2 Al Cl Fe Br Al F Pb
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Answer cathode Possible reactions 2 Pb aet 2 Fe Ret 34 Al 3e 4 SO Pb I E 0126 V Fe E 044 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Marc Loudon
5th edition
981519431, 978-0981519449, 098151944X, 978-0-98151943, 978-0981519432
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



