Answered step by step
Verified Expert Solution
Question
1 Approved Answer
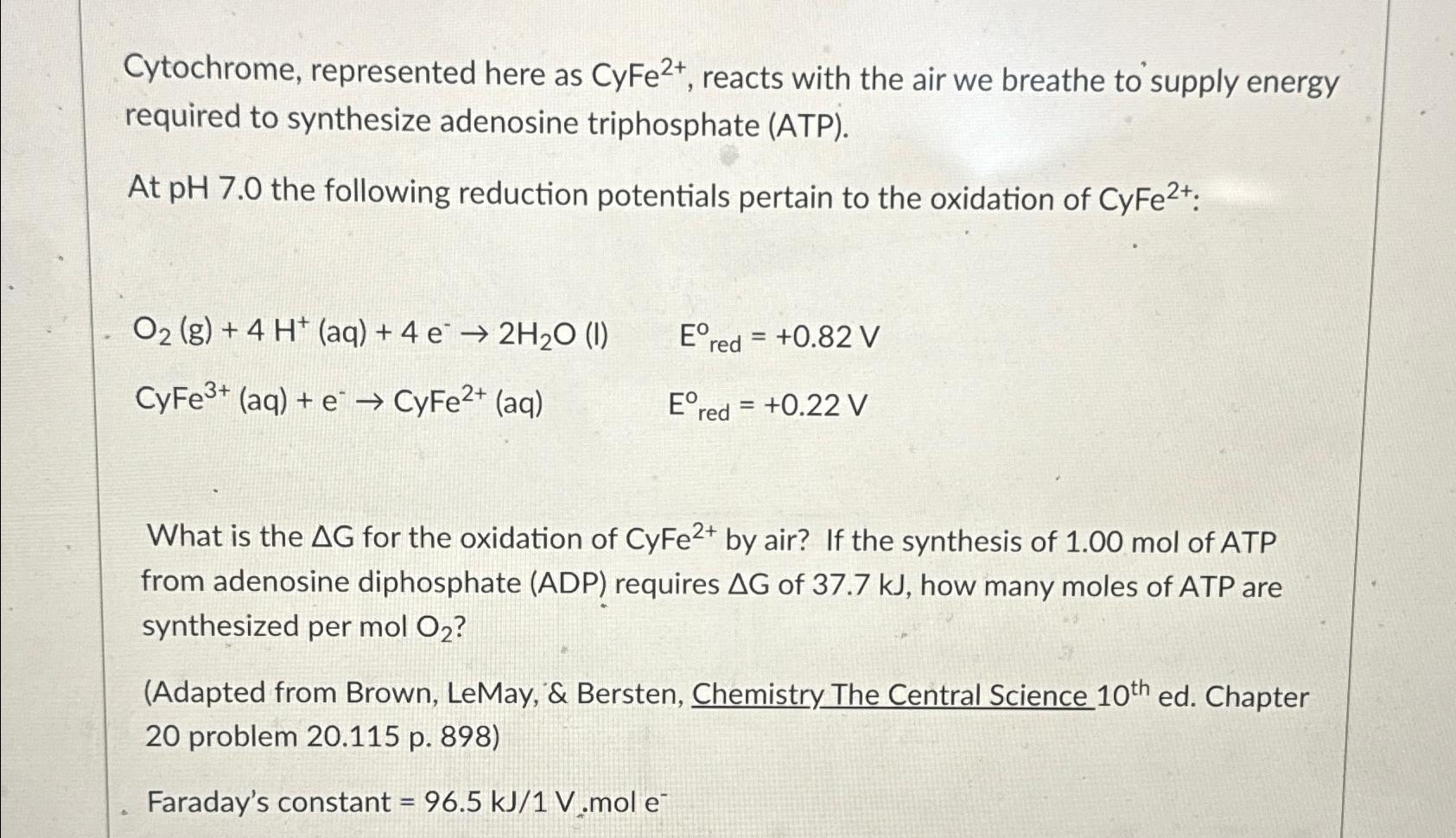
Cytochrome, represented here as C y F e 2 + , reacts with the air we breathe to supply energy required to synthesize adenosine triphosphate
Cytochrome, represented here as reacts with the air we breathe to supply energy required to synthesize adenosine triphosphate ATP
At the following reduction potentials pertain to the oxidation of :
What is the for the oxidation of by air? If the synthesis of mol of ATP from adenosine diphosphate ADP requires of how many moles of ATP are synthesized per
Adapted from Brown, LeMay, & Bersten, Chemistry The Central Science ed Chapter problem p
Faraday's constant
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


