Answered step by step
Verified Expert Solution
Question
1 Approved Answer
D2L Topic 6 Practice with solutions X D2L Quizzes - PHY1321*[B] Princip x Course Hero C x DW 6. The Psychology of the Flood
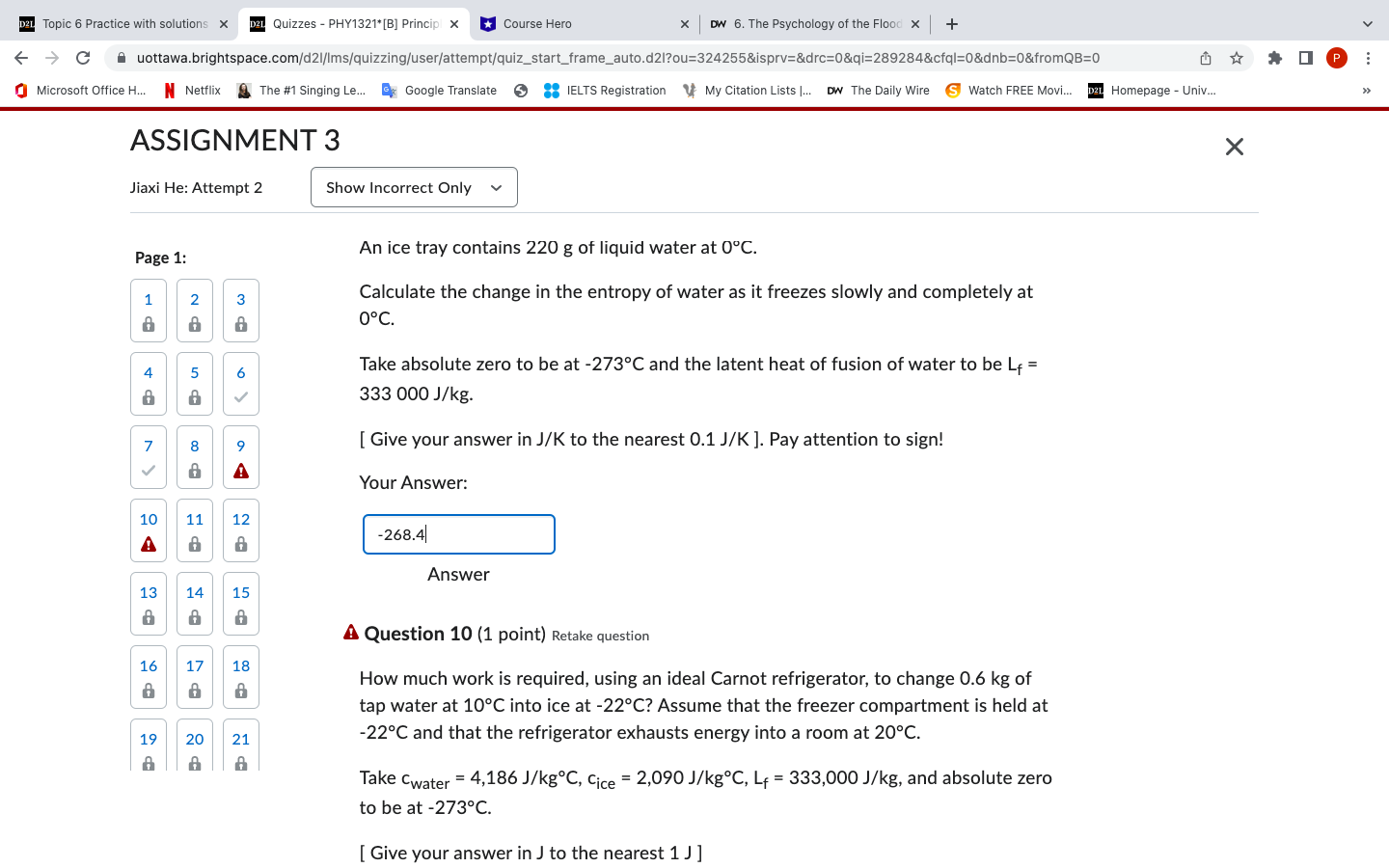
D2L Topic 6 Practice with solutions X D2L Quizzes - PHY1321*[B] Princip x Course Hero C x DW 6. The Psychology of the Flood x + uottawa.brightspace.com/d21/Ims/quizzing/user/attempt/quiz_start_frame_auto.d2l?ou=324255&isprv=&drc=0&qi=289284&cfql=0&dnb=0&fromQB=0 Microsoft Office H... Netflix The #1 Singing Le... Google Translate > :: IELTS Registration My Citation Lists ... DW The Daily Wire Watch FREE Movi... D2L Homepage - Univ... ASSIGNMENT 3 Jiaxi He: Attempt 2 Show Incorrect Only Page 1: 2 8 18 58 4 3 w 6 7 8 9 > 10 A a 1 A 11 12 20 21 8 8 An ice tray contains 220 g of liquid water at 0C. Calculate the change in the entropy of water as it freezes slowly and completely at 0C. Take absolute zero to be at -273C and the latent heat of fusion of water to be L+ = 333 000 J/kg. [ Give your answer in J/K to the nearest 0.1 J/K]. Pay attention to sign! Your Answer: -268.4| Answer 13 14 15 8 8 28 17 18 8 128 16 18 19 28 20 21 21 A Question 10 (1 point) Retake question How much work is required, using an ideal Carnot refrigerator, to change 0.6 kg of tap water at 10C into ice at -22C? Assume that the freezer compartment is held at -22C and that the refrigerator exhausts energy into a room at 20C. Take water = 4,186 J/kgC, Cice = 2,090 J/kgC, L = 333,000 J/kg, and absolute zero to be at -273C. [ Give your answer in J to the nearest 1 J] P :
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


