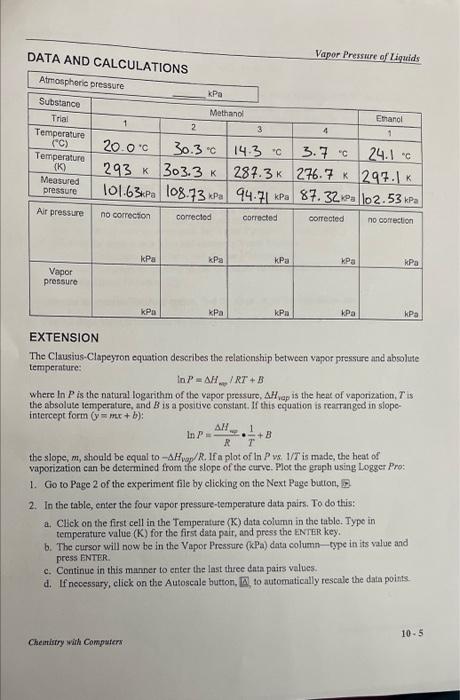
DATA AND CALCULATIONS Vapor Pressure of Liquids EXTENSION The Clausius-Clapeyron equation describes the relationship between vapor pressure and absolute temperature: lnP=H/RT+B where lnP is the natunal logarithm of the vapor paessure, AHapap is the heat of vaporization, T is the absolute temperature, and B is a posidive constant. If this equation is rearanged in slopeintereept form (y=mx+b) : lnP=RH=T1+B the slope, m, should be equal to Hvap/R. If a plot of lnP vs. 1/T is made, the heat of vaporization can be determined from the slope of the curve. Plot the graph using Logger Pro: 1. Go to Page 2 of the experiment file by clicking on the Next Page bution, 2 . 2. In the table, enter the four vapor pressure-temperature data pairs. To do this: a. Click on the first cell in the Temperature (K) data column in the table. Typo in temperature value (K) for the first data pair, and press the ENTER key. b. The cursor will now be in the Vapor Pressure ( kPa ) dati column - type in its value and press ENTER. c. Continue in this manner to enter the last three data pairs values. d. If necessary, cliek on the Autoscale button, [ 8 , to automatically rescale the data poins. Cheirlirry wiin Computers: 105 DATA AND CALCULATIONS Vapor Pressure of Liquids EXTENSION The Clausius-Clapeyron equation describes the relationship between vapor pressure and absolute temperature: lnP=H/RT+B where lnP is the natunal logarithm of the vapor paessure, AHapap is the heat of vaporization, T is the absolute temperature, and B is a posidive constant. If this equation is rearanged in slopeintereept form (y=mx+b) : lnP=RH=T1+B the slope, m, should be equal to Hvap/R. If a plot of lnP vs. 1/T is made, the heat of vaporization can be determined from the slope of the curve. Plot the graph using Logger Pro: 1. Go to Page 2 of the experiment file by clicking on the Next Page bution, 2 . 2. In the table, enter the four vapor pressure-temperature data pairs. To do this: a. Click on the first cell in the Temperature (K) data column in the table. Typo in temperature value (K) for the first data pair, and press the ENTER key. b. The cursor will now be in the Vapor Pressure ( kPa ) dati column - type in its value and press ENTER. c. Continue in this manner to enter the last three data pairs values. d. If necessary, cliek on the Autoscale button, [ 8 , to automatically rescale the data poins. Cheirlirry wiin Computers: 105