Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Data: Coffee type: Sumatra Dark Roast, Mass of water: 600 gram, Mass of beans: 40 grams, Rbrew: 15, pH of coffee at end of brew:6.3
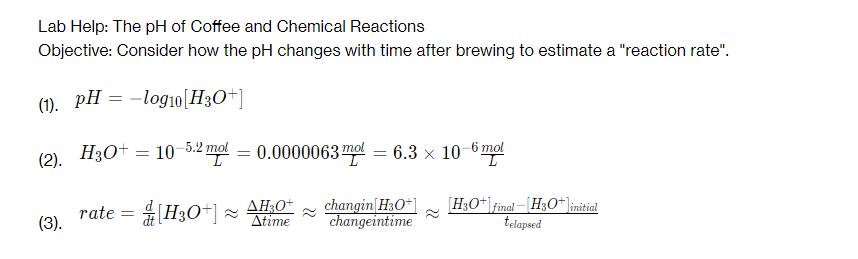
Data:
Coffee type: Sumatra "Dark Roast", Mass of water: 600 gram, Mass of beans: 40 grams, Rbrew: 15, pH of coffee at end of brew:6.3 (This is the pH at time=0).
| Time (minutes) | pH | Time (minutes) | pH |
| 0 | 6.31 | 35 | 5.67 |
| 5 | 6.16 | 40 | 5.61 |
| 10 | 6.00 | 45 | 5.62 |
| 15 | 5.91 | 50 | 5.55 |
| 20 | 5.83 | 55 | 5.54 |
| 25 | 5.79 | 60 | 5.54 |
| 30 | 5.71 |
Sensory Evaluation: t=0 (End of brew): ph=6.31, Bitter, woody, smooth, full body
t=30 minutes: pH=5.71, Acidic
t=60 minutes: pH=5.54, Burnt, acidic
Question: (Please answer and provide explanation)
- First, use Excel to generate a scatter plot of the pH versus time that you generated with the Mr. Coffee. (Put the pH on the vertical axis, and time on the horizontal axis.) What trends do you observe?
- Thank you and will provide good rating.
Lab Help: The pH of Coffee and Chemical Reactions Objective: Consider how the pH changes with time after brewing to estimate a "reaction rate". (1). pH = -log10 [H3O+] (2). H3O+ = 10 5.2 mol = 0.0000063 mol = 6.3 10-6 mol x I rate = (3). [H3O+] AH3O+ changin[H3O+] Atime changeintime [H3O+ final H3O+]initial telapsed
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


