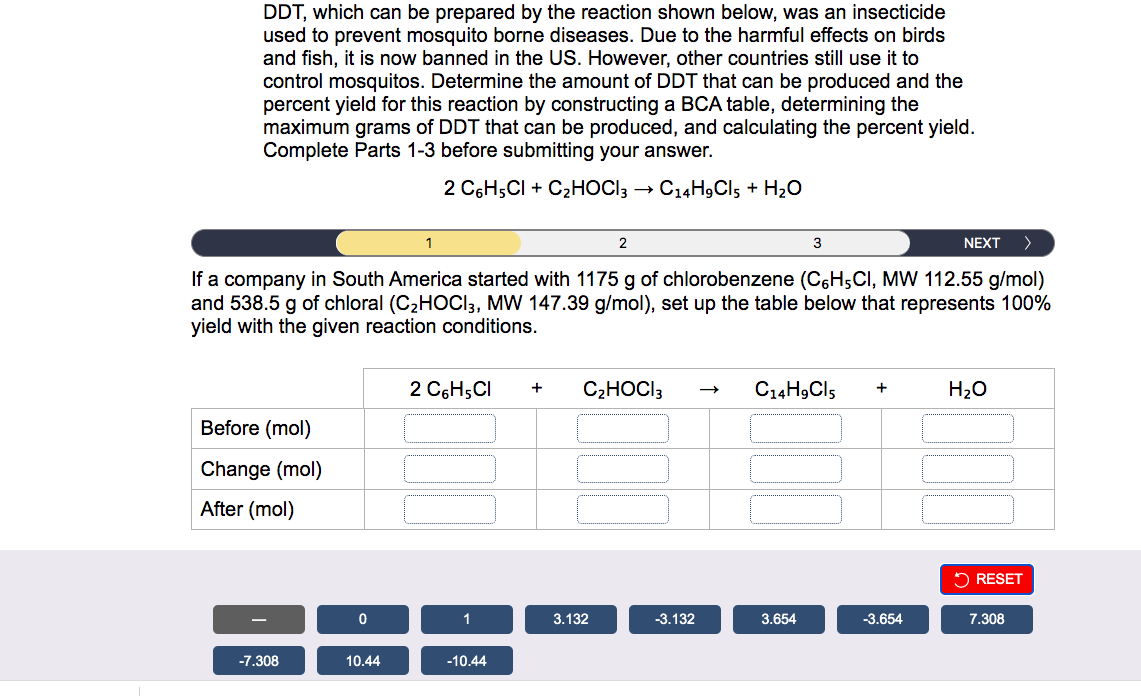
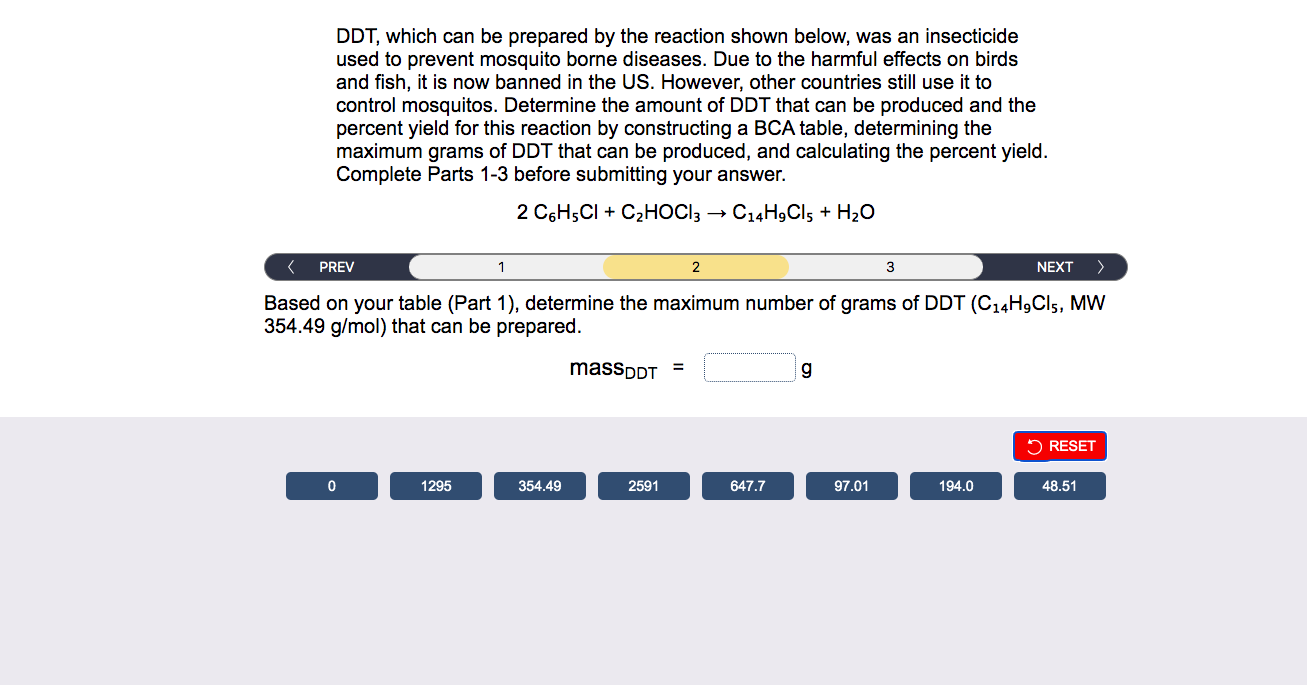
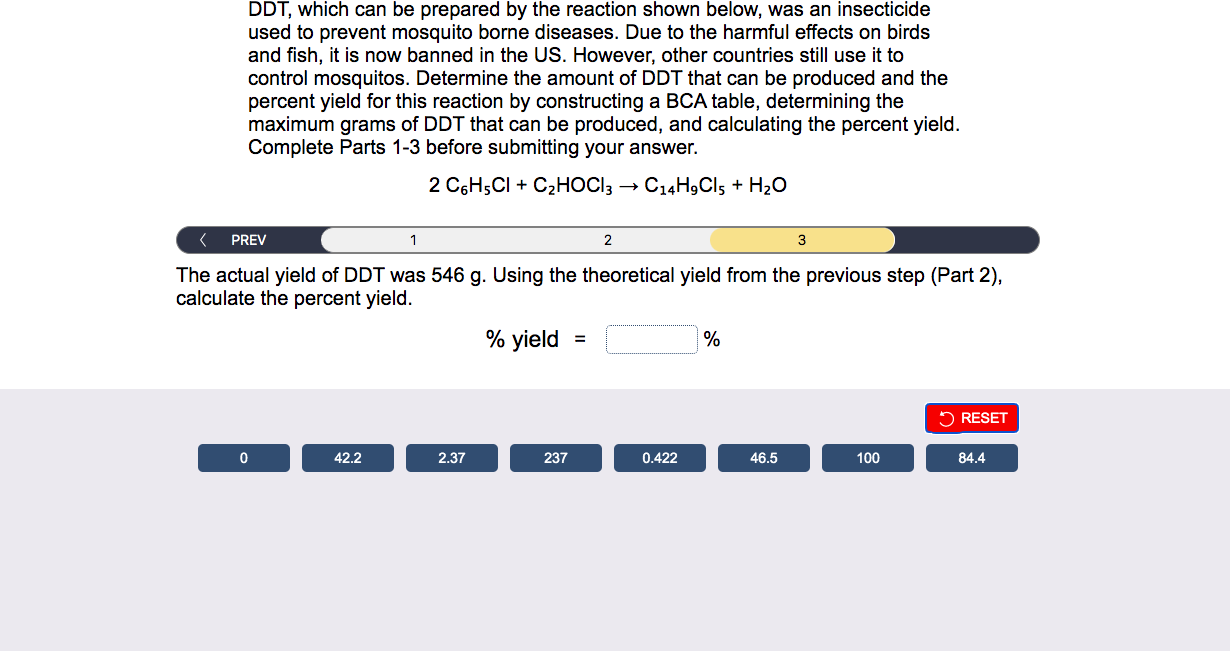
DDT, which can be prepared by the reaction shown below, was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. Determine the amount of DDT that can be produced and the percent yield for this reaction by constructing a BCA table, determining the maximum grams of DDT that can be produced, and calculating the percent yield. Complete Parts 1-3 before submitting your answer. 2C6H5Cl+C2HOCl3C14H9Cl5+H2O If a company in South America started with 1175g of chlorobenzene (C6H5Cl,MW112.55g/mol) and 538.5g of chloral (C2HOCl3,MW147.39g/mol), set up the table below that represents 100% yield with the given reaction conditions. DDT, which can be prepared by the reaction shown below, was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. Determine the amount of DDT that can be produced and the percent yield for this reaction by constructing a BCA table, determining the maximum grams of DDT that can be produced, and calculating the percent yield. Complete Parts 1-3 before submitting your answer. 2C6H5Cl+C2HOCl3C14H9Cl5+H2O > \\ \hline \end{tabular} Based on your table (Part 1), determine the maximum number of grams of DDT (C14H9Cl5,MW 354.49g/mol ) that can be prepared. massDDT=g DDT, which can be prepared by the reaction shown below, was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. Determine the amount of DDT that can be produced and the percent yield for this reaction by constructing a BCA table, determining the maximum grams of DDT that can be produced, and calculating the percent yield. Complete Parts 1-3 before submitting your answer. 2C6H5Cl+C2HOCl3C14H9Cl5+H2O The actual yield of DDT was 546g. Using the theoretical yield from the previous step (Part 2), calculate the percent yield. %yield=%









