Question
Slater's Rule | 0.35 d and f electrons 1.00 1.00 s and p electrons 0.35 0.85 1.00 1.00 1.00 n-3 n-2 n-1 n What
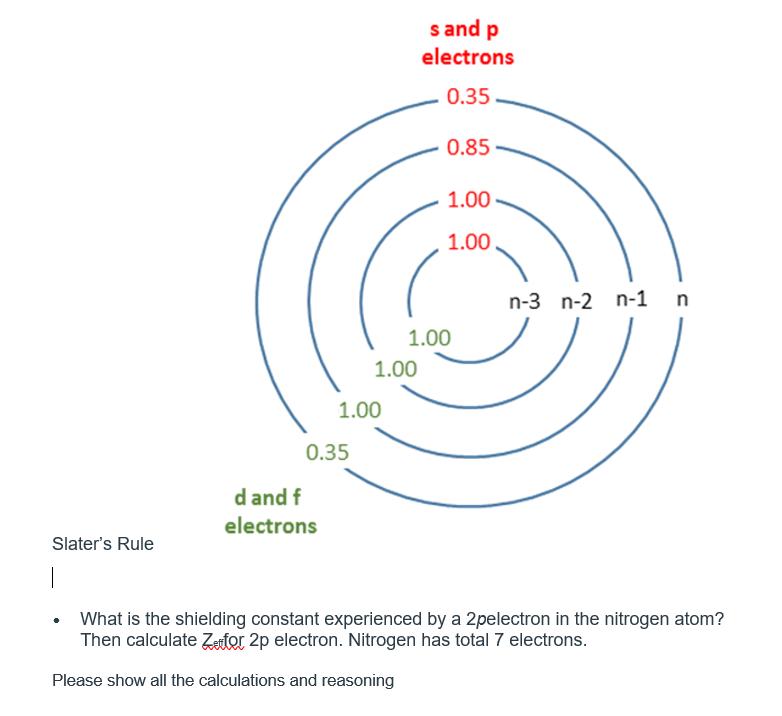
Slater's Rule | 0.35 d and f electrons 1.00 1.00 s and p electrons 0.35 0.85 1.00 1.00 1.00 n-3 n-2 n-1 n What is the shielding constant experienced by a 2pelectron in the nitrogen atom? Then calculate Zetor 2p electron. Nitrogen has total 7 electrons. Please show all the calculations and reasoning
Step by Step Solution
3.47 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
Effective Nuclear Charge Inner e will repell outer due to same charge Due to this Rep...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Auditing and Assurance Services
Authors: Timothy Louwers, Robert Ramsay, David Sinason, Jerry Straws
6th edition
978-1259197109, 77632281, 77862341, 1259197107, 9780077632281, 978-0077862343
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



