Answered step by step
Verified Expert Solution
Question
1 Approved Answer
+ 5 Br (aq) 0.00200M 1. Consider this reaction and the experimental quantities given in the table. BrO3 (aq) + 6 H+ (aq) 3
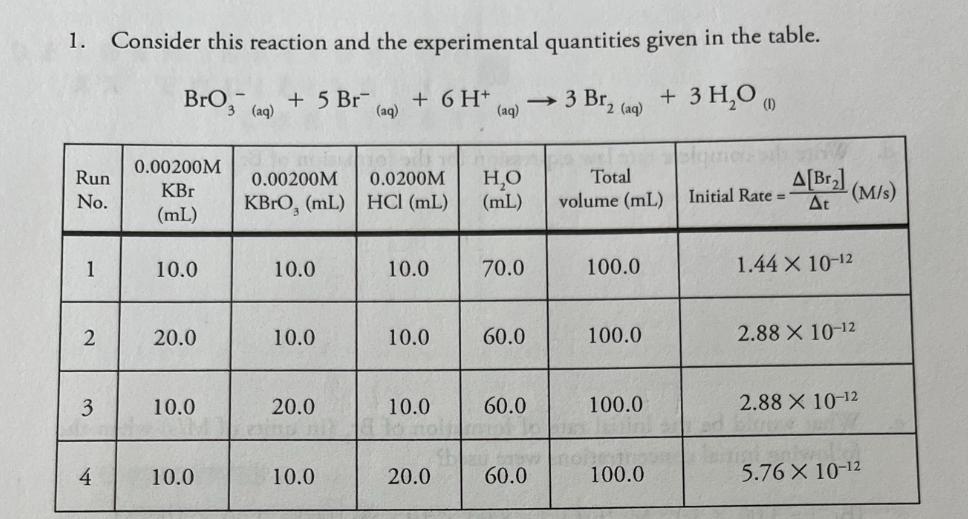
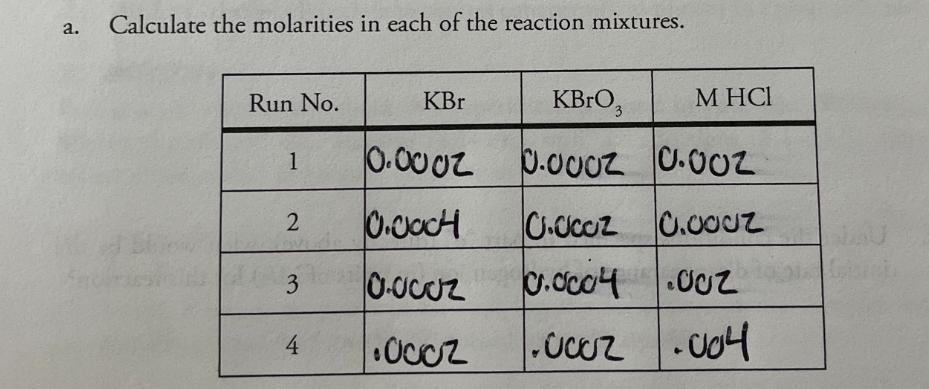
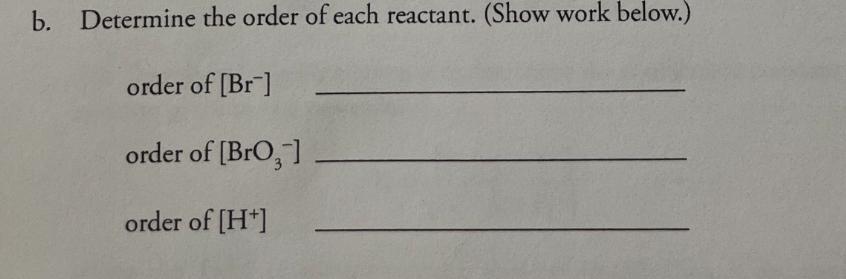

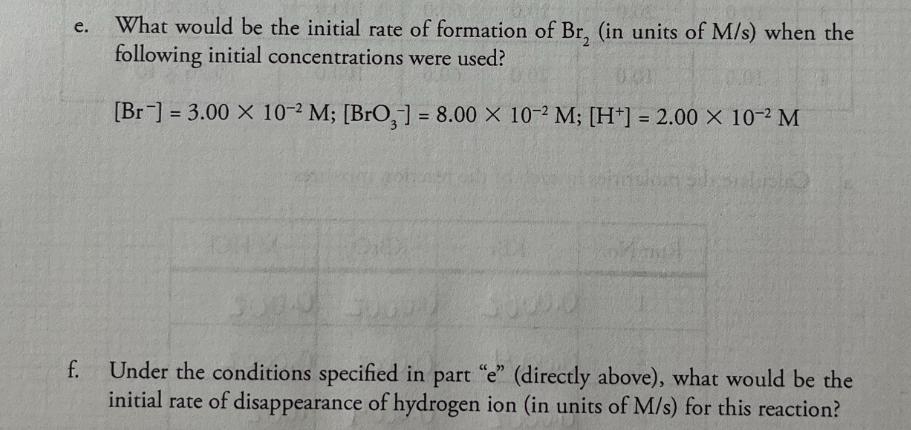
+ 5 Br (aq) 0.00200M 1. Consider this reaction and the experimental quantities given in the table. BrO3 (aq) + 6 H+ (aq) 3 Br, 2 (aq) + 3 HO (1) Run 0.00200M 0.0200M HO Total KBr No. KBrO, (mL) HCl (mL) (mL) volume (mL) Initial Rate= A[Br] At (M/s) (mL) 1 10.0 10.0 10.0 70.0 100.0 1.44 X 10-12 2 20.0 10.0 10.0 60.0 100.0 2.88 X 10-12 3 10.0 20.0 10.0 60.0 100.0 2.88 X 10-12 to hol 4 10.0 10.0 20.0 60.0 100.0 5.76 X 10-12 a. Calculate the molarities in each of the reaction mixtures. Run No. KBr KBrO3 M HCl 1 0.0002 0.0002 0.002 2 0.0004 0.0002 0.0002 3 0.0002 0.0004 .002 0.0004.002 4 .0002 .0002 .004 b. Determine the order of each reactant. (Show work below.) order of [Br] order of [BrO3] order of [H+] C. Determine the value of the rate constant (including units) for the formation of Br. d. Write the complete rate law expression for the formation of Br.. e. What would be the initial rate of formation of Br, (in units of M/s) when the following initial concentrations were used? [Br] =3.00 x 10-2 M; [BrO,] = 8.00 x 10-2 M; [H+] = 2.00 x 10-2 M f. Under the conditions specified in part "e" (directly above), what would be the initial rate of disappearance of hydrogen ion (in units of M/s) for this reaction?
Step by Step Solution
★★★★★
3.36 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
b To determine the order of each reactant we can use the method of initial rates By comparing the in...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


