Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Describe how the three indicator solutions worked in the titrations. Which indicator solution is the best for acid-strong base titration? specify color changes and the
Describe how the three indicator solutions worked in the titrations. Which indicator solution is the best for acid-strong base titration? specify color changes and the pH readings where the color changes occurred.
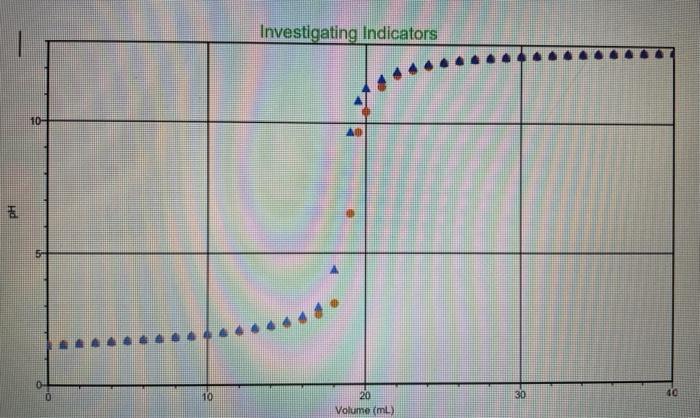
The three indicators are phenolphthalein, bromthymol blue, and methyl orange.
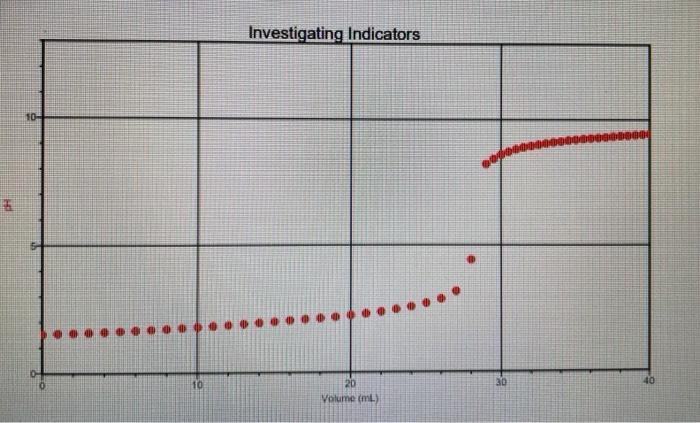
The red graph is with methyl orange.
The orange graph is bromthymol blue.
The blue graph is phenolphthalein.
Investigating Indicators 10- 5- 40 20 Volume (mL) 10 30
Step by Step Solution
★★★★★
3.34 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
Solution The Usree indicaters are Phenopehalein 2 Mettyl orange Bromo lt ymol blue 3 used in titatio...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


