Answered step by step
Verified Expert Solution
Question
1 Approved Answer
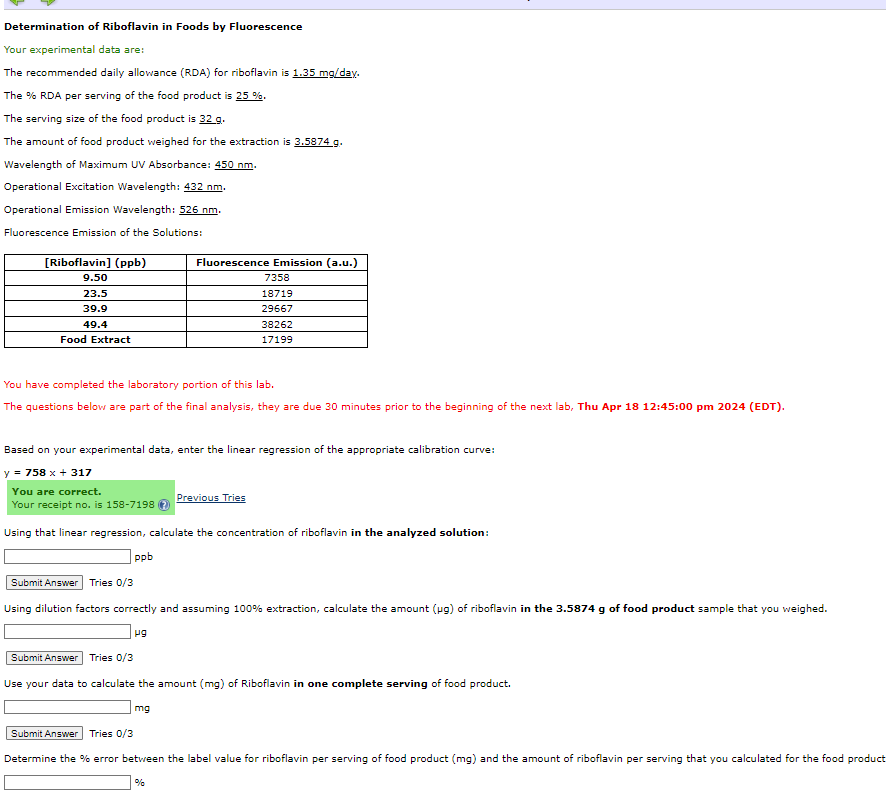
Determination of Riboflavin in Foods by Fluorescence Your experimental data are: The recommended daily allowance ( RDA ) for riboflavin is 1 . 3 5
Determination of Riboflavin in Foods by Fluorescence
Your experimental data are:
The recommended daily allowance RDA for riboflavin is day.
The RDA per serving of the food product is
The serving size of the food product is
The amount of food product weighed for the extraction is
Wavelength of Maximum UV Absorbance:
Operational Excitation Wavelength:
Operational Emission Wavelength:
Fluorescence Emission of the Solutions:
You have completed the laboratory portion of this lab.
The questions below are part of the final analysis, they are due minutes prior to the beginning of the next lab, Thu Apr :: pm EDT
Based on your experimental data, enter the linear regression of the appropriate calibration curve:
You are correct.
Your receipt no is Previous Tries
Using that linear regression, calculate the concentration of riboflavin in the analyzed solution:
pb
Tries
Using dilution factors correctly and assuming extraction, calculate the amount of riboflavin in the of food product sample that you weighed.
Tries
Use your data to calculate the amount of Riboflavin in one complete serving of food product.
Tries
Determine the error between the label value for riboflavin per serving of food product and the amount of riboflavin per serving that you calculated for the food product
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


