Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the atomic number and mass number for each isotope. Part C atomic number for the chromium isotope with 26 neutrons Express your answer


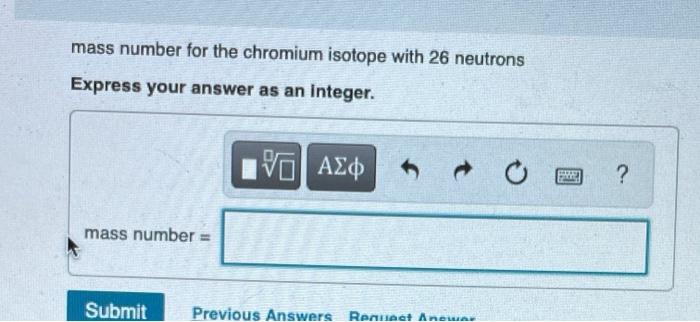
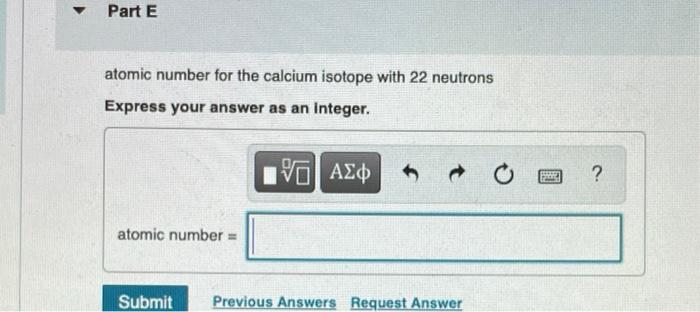

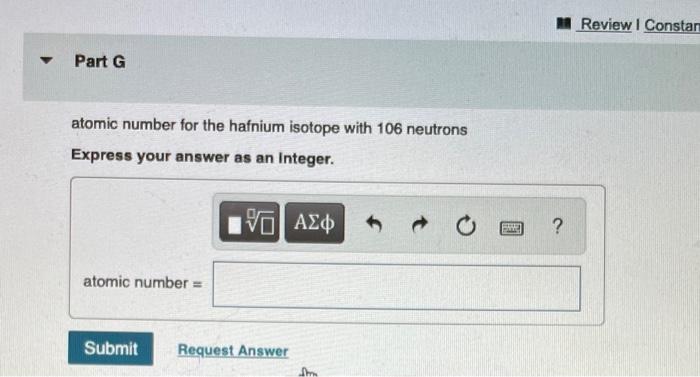

Determine the atomic number and mass number for each isotope. Part C atomic number for the chromium isotope with 26 neutrons Express your answer as an integer. I atomic number = 15. VO Submit Previous Answers Request Answer SE ? mass number for the chromium isotope with 26 neutrons Express your answer as an integer. mass number = 15| Submit Previous Answers Request Anewar SREE ? Part E atomic number for the calcium isotope with 22 neutrons Express your answer as an integer. atomic number = IVE Submit Previous Answers Request Answer ? Part F mass number for the calcium isotope with 22 neutrons Express your answer as an integer. mass number = 15. Submit Request Answer E ? Part G atomic number for the hafnium isotope with 106 neutrons Express your answer as an Integer. atomic number = 15] Submit Request Answer F ? Review I Constar Part H mass number for the hafnium isotope with 106 neutrons Express your answer as an integer. mass number = Submit Provide Feedback 15. Request Answer PAN ?
Step by Step Solution
★★★★★
3.55 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
we know that for an element atomic number Shows the no of protons and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


