Question
Determine the cell reaction and Ecell for each of the following cells at 25 C. Assume activity and fugacity equal to molar concentration and
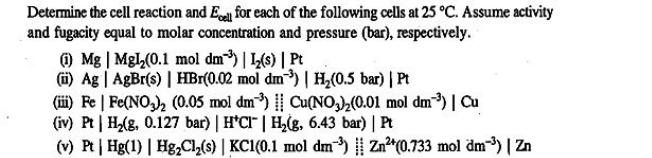
Determine the cell reaction and Ecell for each of the following cells at 25 C. Assume activity and fugacity equal to molar concentration and pressure (bar), respectively. (1) Mg | Mgl(0.1 mol dm) | 1(s) | Pt (i) Ag | AgBr(s) | HBr(0.02 mol dm3) | H,(0.5 bar) | Pt (iii) Fe | Fe(NO3) (0.05 mol dm) | Cu(NO3)(0.01 mol dm) | Cu (iv) Pt | H(g, 0.127 bar) | HCI | H(g, 6.43 bar) | Pt (v) Pt | Hg(1) | HgCl(s) | KC1(0.1 mol dm) | Zn+(0.733 mol dm) | Zn
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



