Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the change in molar Gibbs Free Energy of liquid water at 25C as pressure increased from 1 bar to 10 bar. Repeat for

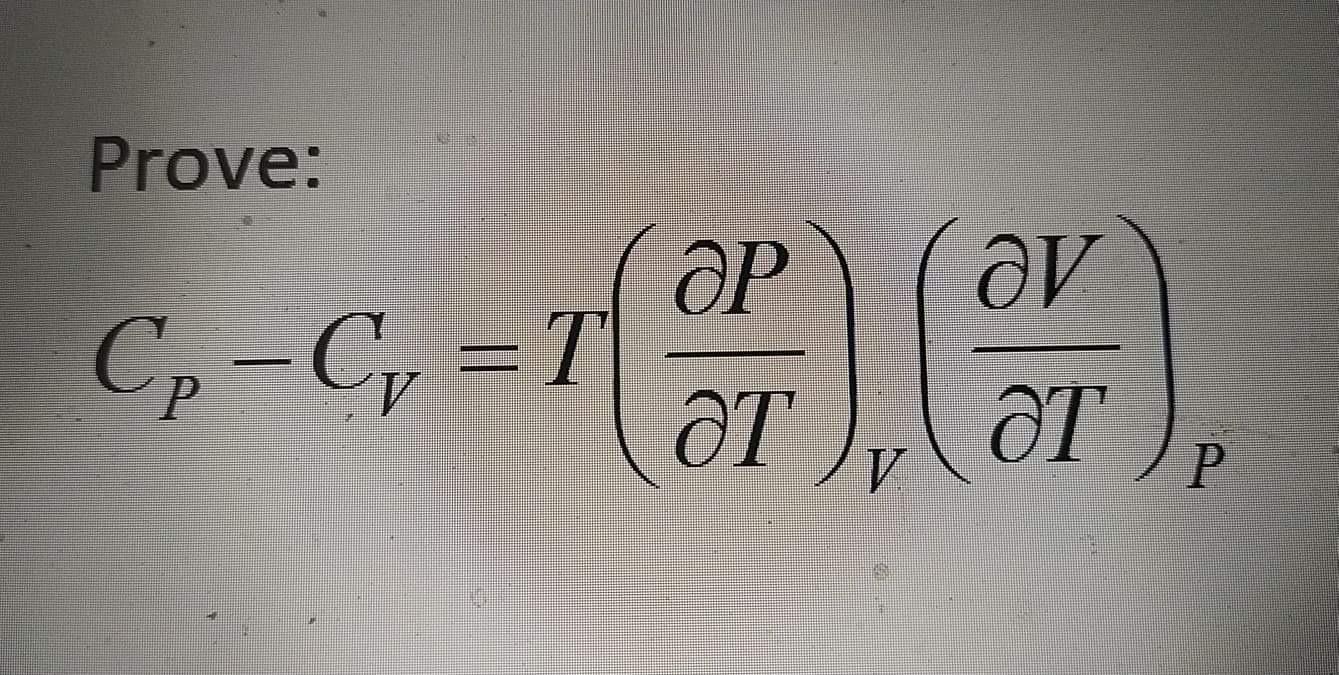
Determine the change in molar Gibbs Free Energy of liquid water at 25C as pressure increased from 1 bar to 10 bar. Repeat for steam assuming ideal gas under same condition. Prove: Cp-C,=DT T
Step by Step Solution
★★★★★
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
636b9f7710cb7_242444.pdf
180 KBs PDF File
636b9f7710cb7_242444.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


