Question
Determine the complete rate equation in units of moles, liters, and seconds for the thermal decomposition of tetrahydrofuran from the half-life data in the
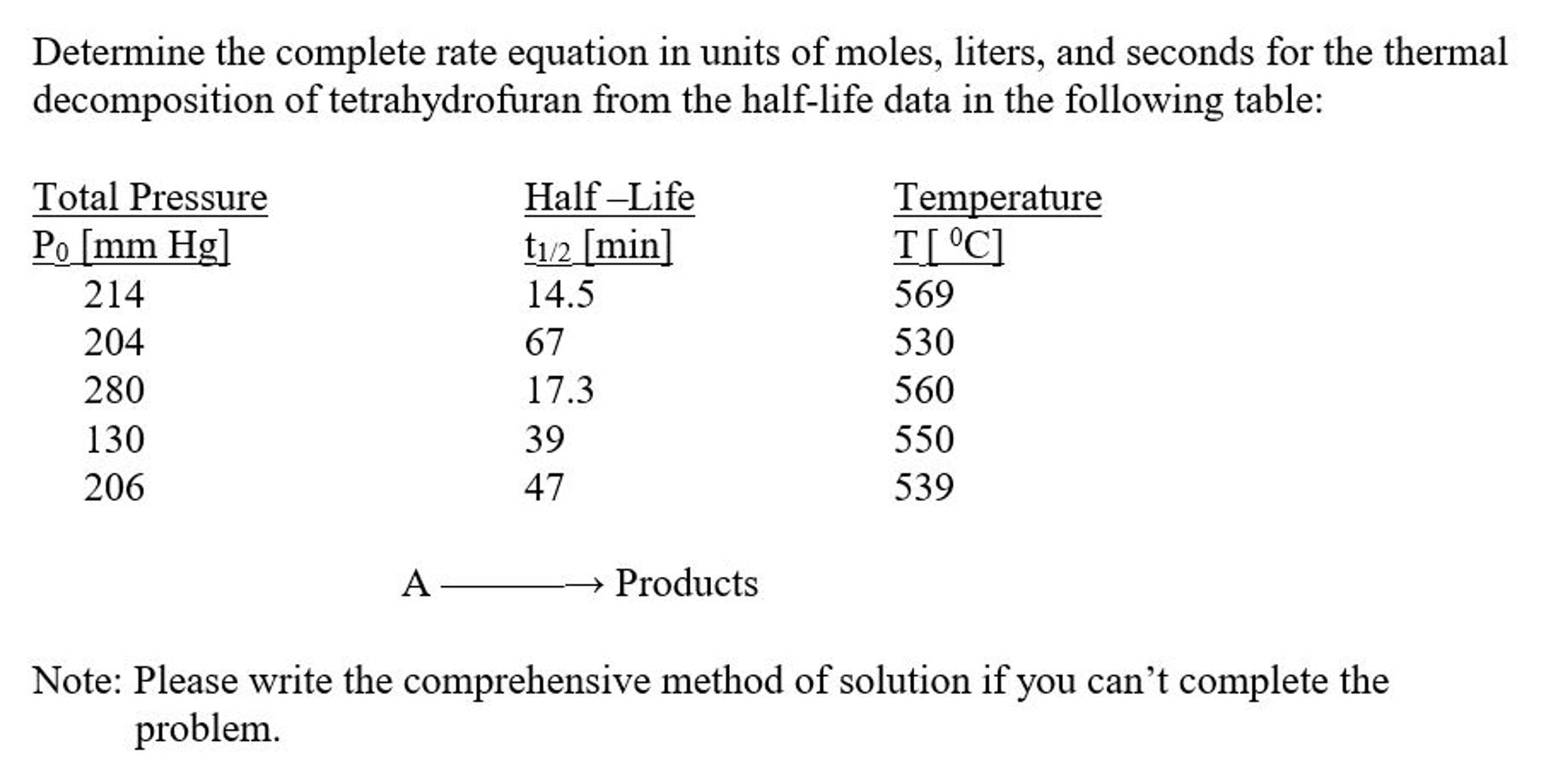
Determine the complete rate equation in units of moles, liters, and seconds for the thermal decomposition of tetrahydrofuran from the half-life data in the following table: Total Pressure Po [mm Hg] Half -Life t12 [min] Temperature T[ C] 214 14.5 569 204 67 530 280 17.3 560 130 39 550 206 47 539 Products Note: Please write the comprehensive method of solution if you can't complete the problem.
Step by Step Solution
3.48 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Given reaction The reaction rate equation As we know Let be equation 1 be equ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Vector Mechanics for Engineers Statics and Dynamics
Authors: Ferdinand Beer, E. Russell Johnston, Jr., Elliot Eisenberg, William Clausen, David Mazurek, Phillip Cornwell
8th Edition
73212229, 978-0073212227
Students also viewed these Economics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



