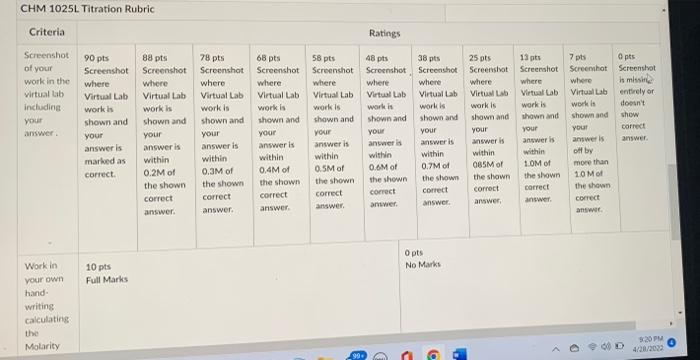
Determine the concentration of an unknown HCI solution using NaOH In this lab you will use a virtual lab to determine the concentration of a strong monoprotic acid by performing a titration. Monoprotic means the acid can only lose 1 H+ (the acid part of the compound.) When you have completed the lab and entered the correct concentration of the acid at the bottom of the page, take a screenshot of the virtual lab page and submit it here for credit. There is no limit on how many times you do the lab, but only 1 submission is allowed. If you have trouble getting the correct answer, you may submit a screenshot of your answer showing it's close to the correct answer (within 0.2M of the correct answer) for most of the points. The rubric below contains details of what you need to submit and the points associated with a wide range of accuracies as well as the requirement to show your work in your own hand-writing (a phone pic of this is OK for this part only). e Please note that once you have prepared a sample for a titration, you may duplicate it as many times as you want to avoid repeating the preparation steps for the same solution. To do this duplication, Right click on the solution and highlight 'duplicate. Right click again to rename your sample. ve Here's a link to a YouTube video about how to use the ChemCollective Virtual Lab. Virtual Strong Acids and Strong Bases Titration Lab Hints on how to do the lab: 4F > Here's a link to a YouTube video about how to use the ChemCollective Virtual Lab. Virtual Strong Acids and Strong Bases Titration Lab Hints on how to do the lab: 1.Start by diluting the initial NaOH solution to 2.00M and using that to titrate 30.00mL of the acid solution (with adding 1mL of phenolphthalein to the acid before any NaOH is added). To dilute the NaOH, combine 400 mL of water with 100mL of NaOH to make 2.00M NaOH. Then add the NaOH in 0.50mL increments. ne 2. Also recall M= mol/L so a 2.00M solution means 2.00mol/L. If you multiply that by the # L (not mL), you get the # moles of base used to neutralize the acid. When your solution 1s turns pink, # moles base = # moles acid. Then take your # moles acid / L acid in sample to calculate molarity.\ 3. The formula for dilution is M1V1 = M2V2 where M1 = initial molarity, M2 = final molarity, V1 initial volume, and V2 = final volume. = CHM 1025L Titration Rubric Criteria Screenshot of your work in the 90 pts Screenshot where Virtual Lab virtual lab including your answer. work is shown and your answer is marked as correct. Work in your own 10 pts Full Marks hand- writing calculating the Molarity 88 pts Screenshot where Virtual Lab work is shown and your answer is within 0.2M of the shown correct answer. 78 pts Screenshot where. Virtual Lab work is shown and your answer is within 0.3M of the shown correct answer. 68 pts Screenshot where Virtual Lab work is shown and your answer is within 0.4M of the shown correct answer. Ratings 58 pts Screenshot 48 pts Screenshot where where Virtual Lab Virtual Lab work is shown and your answer is within 0.5M of work is shown and your answer is within 0.6M of the shown correct the shown correct answer. answer. 38 pts Screenshot where Virtual Lab work is shown and your answer is within 0.7M of the shown correct answer. 0 pts No Marks C C P 25 pts Screenshot where Virtual Lab work is shown and your answer is within 085M of the shown correct answer, 13 pts Screenshot where Virtual Lab work is shown and your answer is within 1.0M of the shown correct answer. 7 pts Screenshot where Virtual Lab work is shown and your answer is off by more than 10 Mo the shown correct answer. 0 pts Screenshot is missin entirely or doesn't show correct answer. 9:20 PM 4/28/2022 Determine the concentration of an unknown HCI solution using NaOH In this lab you will use a virtual lab to determine the concentration of a strong monoprotic acid by performing a titration. Monoprotic means the acid can only lose 1 H+ (the acid part of the compound.) When you have completed the lab and entered the correct concentration of the acid at the bottom of the page, take a screenshot of the virtual lab page and submit it here for credit. There is no limit on how many times you do the lab, but only 1 submission is allowed. If you have trouble getting the correct answer, you may submit a screenshot of your answer showing it's close to the correct answer (within 0.2M of the correct answer) for most of the points. The rubric below contains details of what you need to submit and the points associated with a wide range of accuracies as well as the requirement to show your work in your own hand-writing (a phone pic of this is OK for this part only). e Please note that once you have prepared a sample for a titration, you may duplicate it as many times as you want to avoid repeating the preparation steps for the same solution. To do this duplication, Right click on the solution and highlight 'duplicate. Right click again to rename your sample. ve Here's a link to a YouTube video about how to use the ChemCollective Virtual Lab. Virtual Strong Acids and Strong Bases Titration Lab Hints on how to do the lab: 4F > Here's a link to a YouTube video about how to use the ChemCollective Virtual Lab. Virtual Strong Acids and Strong Bases Titration Lab Hints on how to do the lab: 1.Start by diluting the initial NaOH solution to 2.00M and using that to titrate 30.00mL of the acid solution (with adding 1mL of phenolphthalein to the acid before any NaOH is added). To dilute the NaOH, combine 400 mL of water with 100mL of NaOH to make 2.00M NaOH. Then add the NaOH in 0.50mL increments. ne 2. Also recall M= mol/L so a 2.00M solution means 2.00mol/L. If you multiply that by the # L (not mL), you get the # moles of base used to neutralize the acid. When your solution 1s turns pink, # moles base = # moles acid. Then take your # moles acid / L acid in sample to calculate molarity.\ 3. The formula for dilution is M1V1 = M2V2 where M1 = initial molarity, M2 = final molarity, V1 initial volume, and V2 = final volume. = CHM 1025L Titration Rubric Criteria Screenshot of your work in the 90 pts Screenshot where Virtual Lab virtual lab including your answer. work is shown and your answer is marked as correct. Work in your own 10 pts Full Marks hand- writing calculating the Molarity 88 pts Screenshot where Virtual Lab work is shown and your answer is within 0.2M of the shown correct answer. 78 pts Screenshot where. Virtual Lab work is shown and your answer is within 0.3M of the shown correct answer. 68 pts Screenshot where Virtual Lab work is shown and your answer is within 0.4M of the shown correct answer. Ratings 58 pts Screenshot 48 pts Screenshot where where Virtual Lab Virtual Lab work is shown and your answer is within 0.5M of work is shown and your answer is within 0.6M of the shown correct the shown correct answer. answer. 38 pts Screenshot where Virtual Lab work is shown and your answer is within 0.7M of the shown correct answer. 0 pts No Marks C C P 25 pts Screenshot where Virtual Lab work is shown and your answer is within 085M of the shown correct answer, 13 pts Screenshot where Virtual Lab work is shown and your answer is within 1.0M of the shown correct answer. 7 pts Screenshot where Virtual Lab work is shown and your answer is off by more than 10 Mo the shown correct answer. 0 pts Screenshot is missin entirely or doesn't show correct answer. 9:20 PM 4/28/2022