Answered step by step
Verified Expert Solution
Question
1 Approved Answer
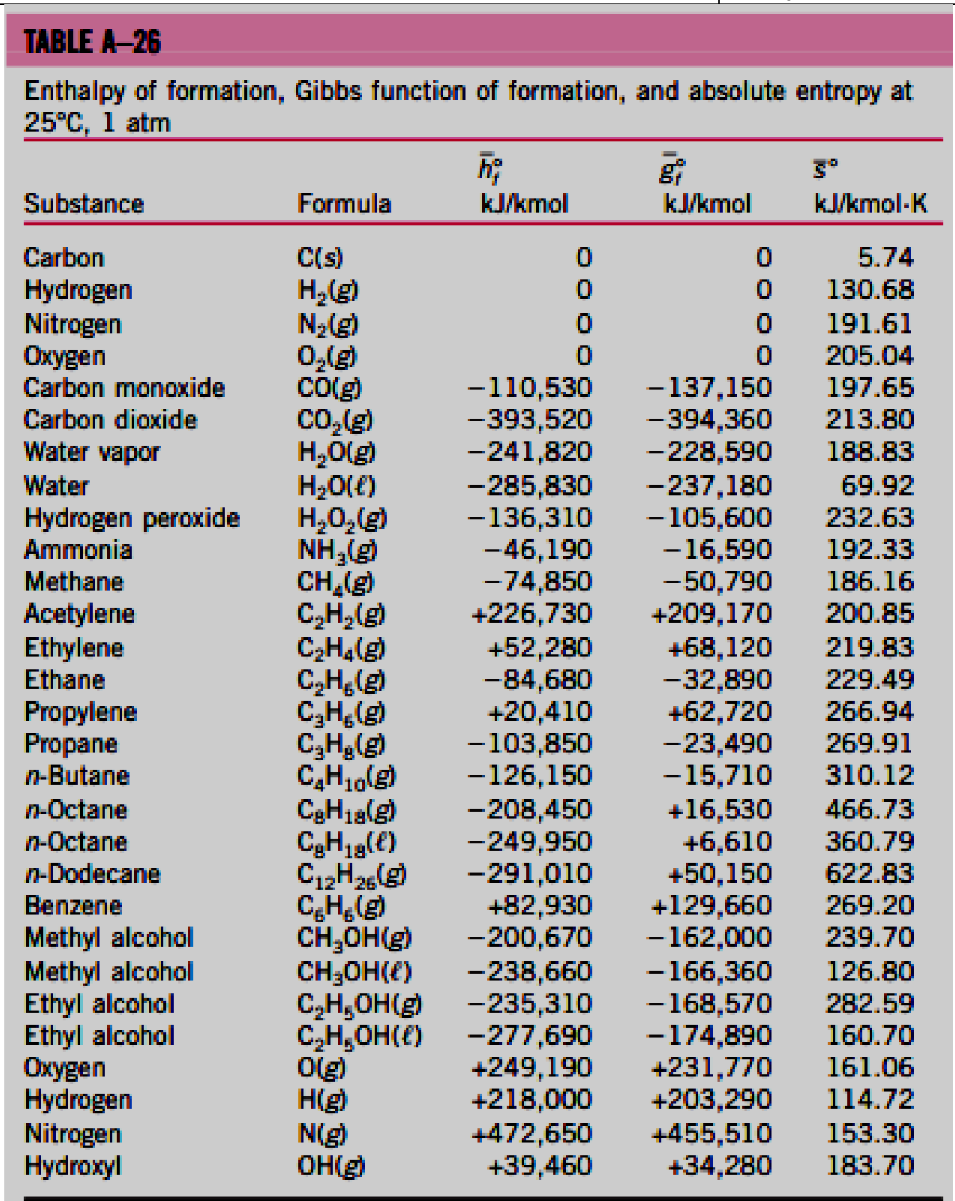
Determine the enthalpy of combustion ( in MJ / kg ) of fuel at 2 5 deg C and 1 atm, using the enthalpy
Determine the enthalpy of combustion in MJkg of fuel at deg C and atm, using the
enthalpy of formation data from Table Aprovided below
Fuel is burned with excess air during a combustion process. Assuming complete
combustion and the water in the products is in the vapor state, determine also the enthalpy
of combustion in MJkg of the mixture, the fuel is Ethane CHgtableTABLE AtableEnthalpy of formatatm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


