Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the heat added or liberated per 100 mole of gas mixture. 1. A gas mixture, of composition: 12.8 mol% CO(g), 3.7 mol% CO2(g), 5.4
Determine the heat added or liberated per 100 mole of gas mixture. 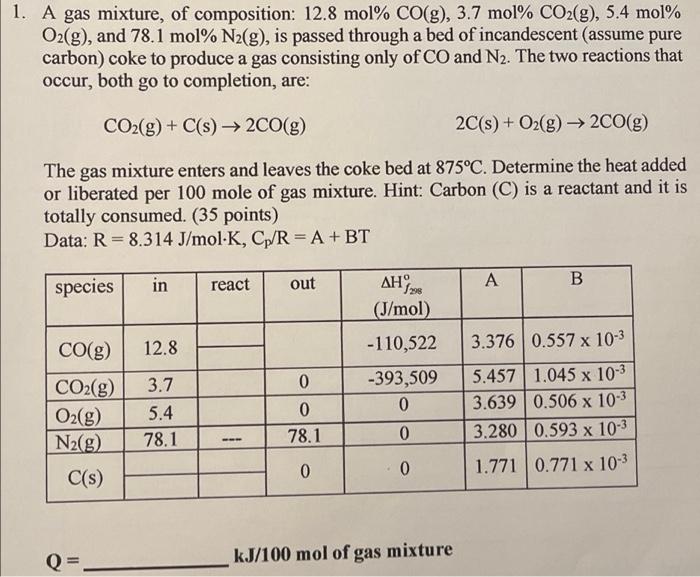
1. A gas mixture, of composition: 12.8 mol% CO(g), 3.7 mol% CO2(g), 5.4 mol% O2(g), and 78.1 mol% N2(g), is passed through a bed of incandescent (assume pure carbon) coke to produce a gas consisting only of CO and N2. The two reactions that occur, both go to completion, are: CO2(g) + C(s) 2CO(g) 2C(s) + O2(g) 2CO(g) The gas mixture enters and leaves the coke bed at 875C. Determine the heat added or liberated per 100 mole of gas mixture. Hint: Carbon (C) is a reactant and it is totally consumed. (35 points) Data: R= 8.314 J/mol K, Cp/R = A +BT in species react out A B , (J/mol) CO(g) 12.8 3.376 0.557 x 10-3 -110,522 -393,509 0 CO2(g) O2(g) N2(g) 3.7 5.4 78.1 0 0 78.1 5.457 1.045 x 10-3 3.639 0.506 x 10-3 3.280 0.593 x 10-3 1.771 0.771 x 10-3 0 0 0 C(s) Q kJ/100 mol of gas mixture 1. A gas mixture, of composition: 12.8 mol% CO(g), 3.7 mol% CO2(g), 5.4 mol% O2(g), and 78.1 mol% N2(g), is passed through a bed of incandescent (assume pure carbon) coke to produce a gas consisting only of CO and N2. The two reactions that occur, both go to completion, are: CO2(g) + C(s) 2CO(g) 2C(s) + O2(g) 2CO(g) The gas mixture enters and leaves the coke bed at 875C. Determine the heat added or liberated per 100 mole of gas mixture. Hint: Carbon (C) is a reactant and it is totally consumed. (35 points) Data: R= 8.314 J/mol K, Cp/R = A +BT in species react out A B , (J/mol) CO(g) 12.8 3.376 0.557 x 10-3 -110,522 -393,509 0 CO2(g) O2(g) N2(g) 3.7 5.4 78.1 0 0 78.1 5.457 1.045 x 10-3 3.639 0.506 x 10-3 3.280 0.593 x 10-3 1.771 0.771 x 10-3 0 0 0 C(s) Q kJ/100 mol of gas mixture 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


