Question
Determine the isotope symbol that fits each description. a. 68 neutrons, 47 electrons 47 115 c. atomic number = 86, 136 neutrons 86 Ag
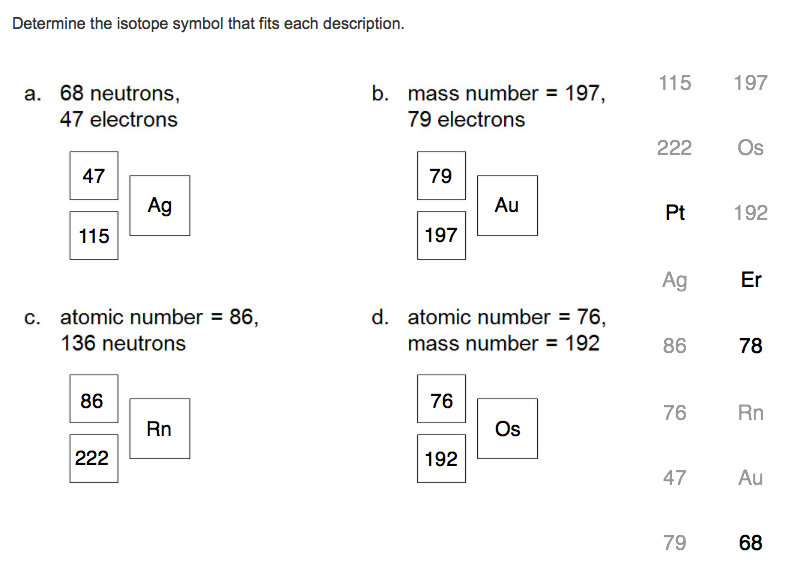
Determine the isotope symbol that fits each description. a. 68 neutrons, 47 electrons 47 115 c. atomic number = 86, 136 neutrons 86 Ag 222 Rn b. mass number = 197, 79 electrons 79 197 d. atomic number = 76, mass number = 192 76 Au 192 Os 115 222 Os Pt Ag 86 76 47 197 79 192 Er 78 Rn Au 68
Step by Step Solution
3.52 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
For given isotopes the suitable symbols are shown in the bel...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Biology
Authors: Mary Jones, Richard Fosbery, Jennifer Gregory, Dennis Taylor
4th Edition
1107636825, 978-1107636828
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



