Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the limiting reactant. Justify. Propylene (C3H6) reacts with oxygen and hydrogen to produce Propylene oxide (C3H8O). A side reaction resulted in reacting propylene with
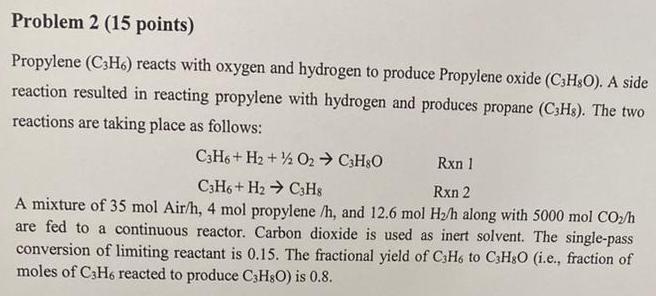
Determine the limiting reactant. Justify.
Propylene (C3H6) reacts with oxygen and hydrogen to produce Propylene oxide (C3H8O). A side reaction resulted in reacting propylene with hydrogen and produces propane (C3H8). The two reactions are taking place as follows: C3H6+H2+1/2O2C3H8OC3H6+H2C3H8Rxn1Rxn2 A mixture of 35molAir/h,4mol propylene /h, and 12.6molH2/h along with 5000molCO/h are fed to a continuous reactor. Carbon dioxide is used as inert solvent. The single-pass conversion of limiting reactant is 0.15 . The fractional yield of C3H6 to C3H8O (i.e., fraction of moles of C3H6 reacted to produce C3H8O ) is 0.8Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


