Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Determine the stoichiometric coefficients for the combustion of PMMA and oxygen (for stoichiometric conditions, d = 0). C502H8a (02) b(CO2)+ c(HO) + d(0) Enthalpy of
Determine the stoichiometric coefficients for the combustion of PMMA and oxygen (for stoichiometric conditions, d = 0).
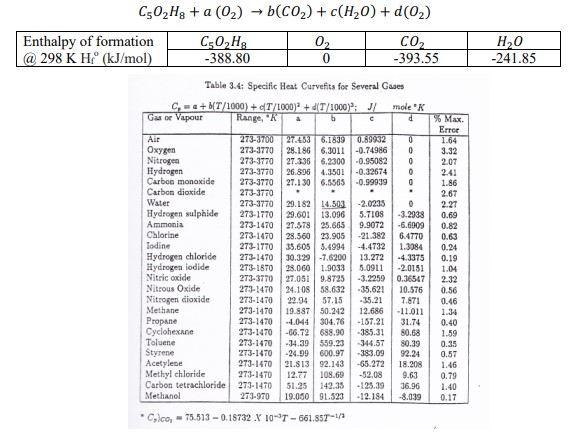
C502H8a (02) b(CO2)+ c(HO) + d(0) Enthalpy of formation @ 298 K Hr (kJ/mol) C502H8 -388.80 -> 02 0 CO -393.55 HO -241.85 Table 3.4: Specific Heat Curvefits for Several Gases C, a+b(T/1000)+(T/1000)+d(T/1000); J/ mole "K Gas or Vapour Range, "K a b d % Max. Error Air 273-3700 Oxygen Nitrogen Hydrogen Carbon monoxide Carbon dioxide 27.453 6.1839 0.89932 273-3770 28.186 6.3011 -0.74986 273-3770 27.336 6.2300 -0.95082 273-3770 26.896 4.3501 -0.32674 273-3770 27.130 6.5565 273-3770 0 1.64 0 3.32 0 2.07 2.41 -0.99939 0 1.86 . . 2.67 Water Hydrogen sulphide Ammonia Chlorine 273-3770 29.182 14.503 273-1770 29.601 13.096 273-1470 27.578 25.665 273-1470 28.560 23.905 -2.0235 0 2.27 5.7108 -3.2938 0.69 9.9072 -6.6909 0.82 -21.382 6.4770 0.63 Iodine Hydrogen chloride 273-1770 273-1470 Hydrogen iodide 273-1870 Nitric oxide 273-3770 Nitrous Oxide Nitrogen dioxide Methane Propane Cyclohexane 35.605 5.4994 30.329 -7.6200 28.060 1.9033 27.051 9.8725 273-1470 24.108 58.632 273-1470 22.94 57.15 273-1470 19.887 50.242 273-1470 -4.044 304.76 273-1470 -66.72 688.90 -385.31 -4.4732 1.3084 0.24 13.272 -4.3375 0.19 5.0911 -2.0151 1.04 -3.2259 0.36547 2.32 -35.621 10.576 -35.21 7.871 0.56 0.46 12.686 -11.011 1.34 -157.21 31.74 0.40 80.68 1.59 Toluene 273-1470 -34.39 559.23 -344.57 80.39 0.35 Styrene 273-1470 -24.99 600.97 -383.09 92.241 0.57 Acetylene 273-1470 21.813 92.143 Methyl chloride 273-1470 12.77 108.69 Carbon tetrachloride 273-1470 51.25 142.35 -125.39 36.96 Methanol 273-970 19.050 91.523 -12.184 -8.039 Clco, 75.513-0.18732 X 10-7-661.857-1/3 -65.272 18.208 1.46 -52.08 9.63 0.79 1.40 0.17
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


