Question
Determine whether a bond between each of the following pairs of atoms would be pure covalent, polar covalent, or ionic. Drag the appropriate items to
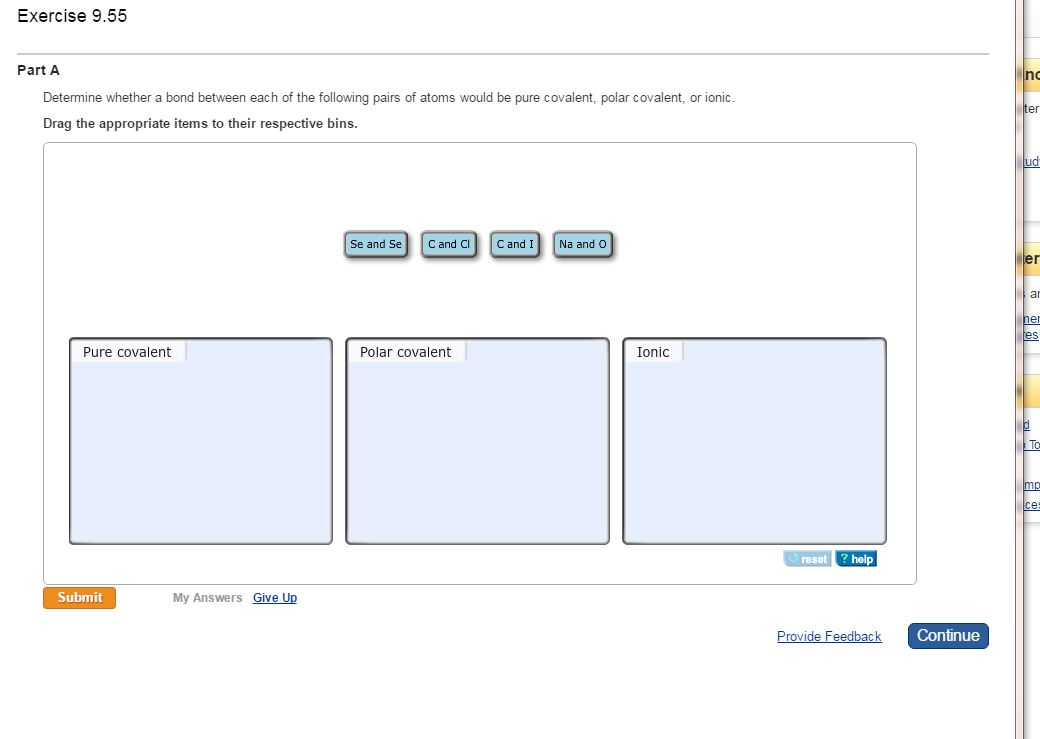
Determine whether a bond between each of the following pairs of atoms would be pure covalent, polar covalent, or ionic.
Drag the appropriate items to their respective bins.
|
Exercise 9.55 Part A Determine whether a bond between each of the following pairs of atoms would be pure covalent, polar covalent, or ionic. Drag the appropriate items to their respective bins. Pure covalent Submit My Answers Give Up Se and Se C and Cl Polar covalent C and I Na and O Ionic reset ? help Provide Feedback Continue no ter ud er To mp ce
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
ionic bond is formed between a metal and a non metal Covalent compoun...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Economics
Authors: Paul Keat, Philip K Young, Steve Erfle
7th edition
0133020266, 978-0133020267
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



