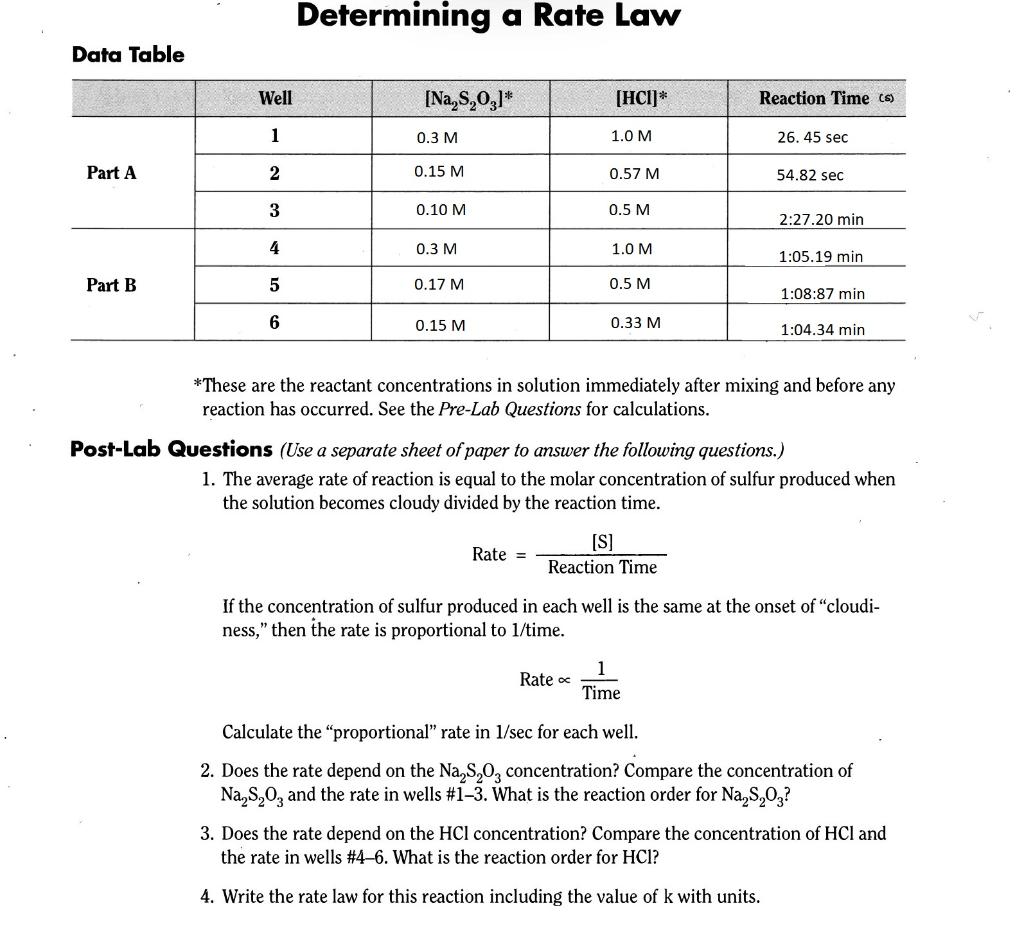
Determining a Rate Law Data Table Well [Na,3,031* [HCI)* Reaction Time (s) 1 0.3 M 1.0 M 26.45 sec Part A 2 0.15 M 0.57 M 54.82 sec 3 0.10 M 0.5 M 2:27.20 min 4 0.3M 1.0 M 1:05.19 min Part B 5 0.17 M 0.5 M 1:08:87 min 6 0.15 M 0.33 M 1:04.34 min *These are the reactant concentrations in solution immediately after mixing and before any reaction has occurred. See the Pre-Lab Questions for calculations. Post-Lab Questions (Use a separate sheet of paper to answer the following questions.) 1. The average rate of reaction is equal to the molar concentration of sulfur produced when the solution becomes cloudy divided by the reaction time. Rate = [S] Reaction Time If the concentration of sulfur produced in each well is the same at the onset of cloudi- ness," then the rate is proportional to 1/time. Rate 1 Time Calculate the proportional" rate in 1/sec for each well. 2. Does the rate depend on the Na,s,o, concentration? Compare the concentration of Na,3,0, and the rate in wells #1-3. What is the reaction order for Na,3,0,? 3. Does the rate depend on the HCl concentration? Compare the concentration of HCl and the rate in wells #4-6. What is the reaction order for HCI? 4. Write the rate law for this reaction including the value of k with units. Determining a Rate Law Data Table Well [Na,3,031* [HCI)* Reaction Time (s) 1 0.3 M 1.0 M 26.45 sec Part A 2 0.15 M 0.57 M 54.82 sec 3 0.10 M 0.5 M 2:27.20 min 4 0.3M 1.0 M 1:05.19 min Part B 5 0.17 M 0.5 M 1:08:87 min 6 0.15 M 0.33 M 1:04.34 min *These are the reactant concentrations in solution immediately after mixing and before any reaction has occurred. See the Pre-Lab Questions for calculations. Post-Lab Questions (Use a separate sheet of paper to answer the following questions.) 1. The average rate of reaction is equal to the molar concentration of sulfur produced when the solution becomes cloudy divided by the reaction time. Rate = [S] Reaction Time If the concentration of sulfur produced in each well is the same at the onset of cloudi- ness," then the rate is proportional to 1/time. Rate 1 Time Calculate the proportional" rate in 1/sec for each well. 2. Does the rate depend on the Na,s,o, concentration? Compare the concentration of Na,3,0, and the rate in wells #1-3. What is the reaction order for Na,3,0,? 3. Does the rate depend on the HCl concentration? Compare the concentration of HCl and the rate in wells #4-6. What is the reaction order for HCI? 4. Write the rate law for this reaction including the value of k with units







