Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider two systems (A and B) under the following conditions: System A B Specific Heat Capacity 1.00 2.00 Mass Initial Temperature 100.0 g 30.0C
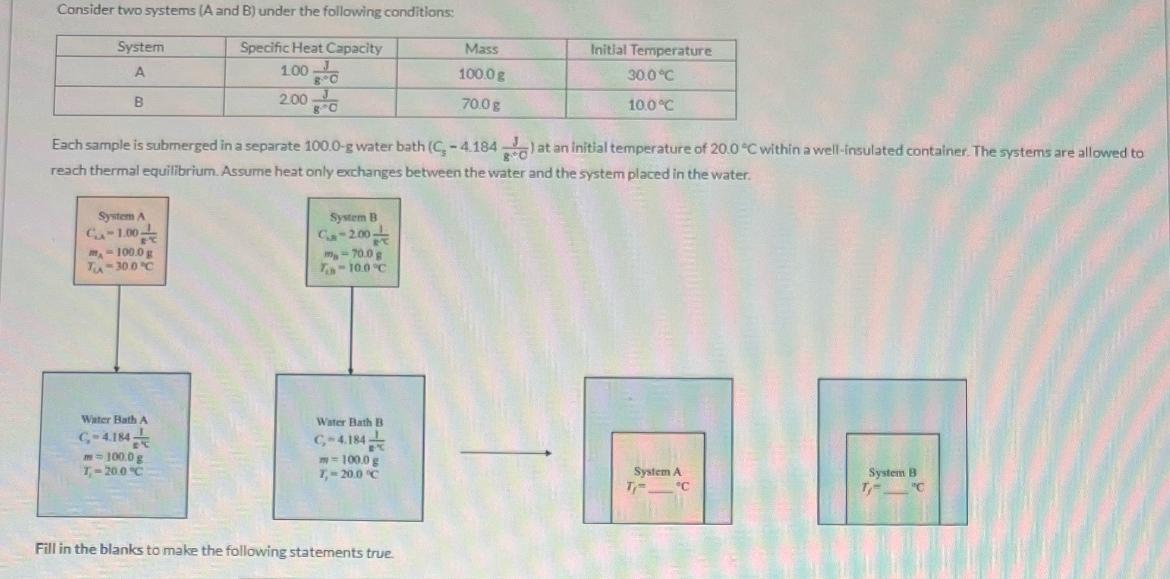
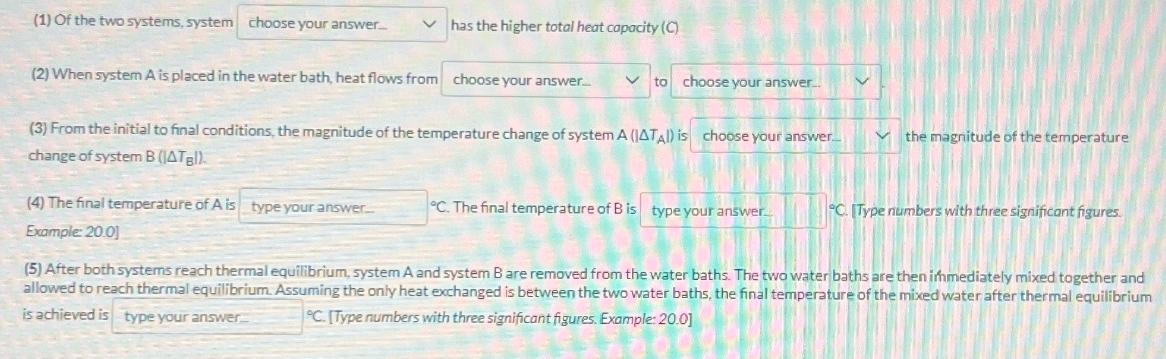
Consider two systems (A and B) under the following conditions: System A B Specific Heat Capacity 1.00 2.00 Mass Initial Temperature 100.0 g 30.0C 70.0 g 10.0C Each sample is submerged in a separate 100.0-g water bath (C-4.184) at an initial temperature of 20.0 C within a well-insulated container. The systems are allowed to reach thermal equilibrium. Assume heat only exchanges between the water and the system placed in the water. System A C-1.00 m-100.0 g Tx-30.0C System B C-2.00 m-70.0 g T-10.0C Water Bath A C-4.184 m-100.0 g T-20.0C Water Bath B C-4.184 m=100.0 g T-20.0 C Fill in the blanks to make the following statements true. System A T- System B C T "C (1) Of the two systems, system choose your answer... has the higher total heat capacity (C) to choose your answer... (2) When system A is placed in the water bath, heat flows from choose your answer.... (3) From the initial to final conditions, the magnitude of the temperature change of system A (IATA) is choose your answer change of system B (ATB). the magnitude of the temperature (4) The final temperature of A is type your answer... Example: 20.01 C. The final temperature of B is type your answer. C. [Type numbers with three significant figures. (5) After both systems reach thermal equilibrium, system A and system B are removed from the water baths. The two water baths are then immediately mixed together and allowed to reach thermal equilibrium. Assuming the only heat exchanged is between the two water baths, the final temperature of the mixed water after thermal equilibrium is achieved is type your answer C. [Type numbers with three significant figures. Example: 20.01
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


