Question
Dilution of the Maalox (milk of magnesia with magnesium hydroxide) Solution 1. Record the brand and %m/v Mg(OH) 2 in your Maalox antacid or generic
Dilution of the Maalox (milk of magnesia with magnesium hydroxide) Solution
1. Record the brand and %m/v Mg(OH)2 in your Maalox antacid or generic form. Note that some brands contain Al(OH)3.
2. Dilute your antacid 1:3 (1 part antacid + 3 parts water) for a total volume to be used in 4-5 titrations (the final volume is for you to determine but in general, making a total of ~30 mL should be enough) (1 teaspoon ~ 5 mL).
Titration of Maalox with Vinegar
1. Record the brand and %v/v of your vinegar and milk of magnesia. Check pH with cabbage indicator for each and take a photo. Record
2. Using a syringe (record; ie. a 3 mL syringe, etc.), dispense 5.0 mL of diluted Maalox into a clean container.
3. Add ~? mL of cabbage juice; mix,
5. Add ~ 4 mL of vinegar, slowly and with stirring until the solution is clear (not colorless); this may take more or less vinegar depending on your brand. Record initial & final volumes to the correct sf.
Run 1 initial volume = 0.0 ml final volume= 4.0 ml
Run 2 initial volume= 0.0 ml final volume = 3.8 ml
Run3 initial volume = 0.0 ml final volume= 4.0 ml
Calculations
1. Show the calculation for:
a. the molarity of acetic acid in your vinegar. Obtain the percent v/v from the label, and use 1.05 g/mL as density (it will probably be to one significant figures but write it as two sf; Ex. 3.0% v/v or acidity rather than 3% v/v or acidity).
2. Show the calculations for the original (or the label) magnesium hydroxide (our accepted magnesium hydroxide, in this scenario):
a. the initial %m/v of magnesium hydroxide (use the label information; it will be in mg/mL ); we will call this %m/v label or accepted. This value will later be used as our accepted value. As above, use two sf even if the label only has one.
b. the initial molarity of the magnesium hydroxide (start with the calculation above); you can call this M1 original or label.
c. diluted molarity of magnesium hydroxide (in your antacid) used in the titration; you can call this M1 diluted,original or label.
3. Show the calculations for our experimental magnesium hydroxide (this is how we test if we obtained the same as it's in the label):
a. the experimental molarity of the diluted magnesium hydroxide for each run, using the titration data; you can call this M2 diluted-experimental-titration.
b. the average experimental molarity of the magnesium hydroxide for all three runs and we will call it M2 diluted-experimental titration(average) ; also calculate the standard deviation.
c. the undiluted average experimental molarity of magnesium hydroxide using the M2 diluted-experimental (average), and the dilution information in calculation 1c above; we will call this M2 undiluted experimental titration (average).
d. the %m/v of the magnesium hydroxide determined from the titration data, using the M2 undiluted experimental (average) value above; we will call this % m/v experimental titration.
4. Did we get the same value as listed on the label? Calculate the percent error for the %m/v of magnesium hydroxide obtained in the titration above. Use the calculation in 2a or %m/v label or accepted as your accepted value (the value from the label), and use the calculation in 3d or % m/v experimental titration as your experimental (the value obtained from the titration).
Brand of Mg(OH)2 = equate
% w/v of Mg(OH)2= 1200mg/15 ml
% v/v of vinegar= 5.0%
Help me out I'm totally confused in calculation and dilutions please help experts please
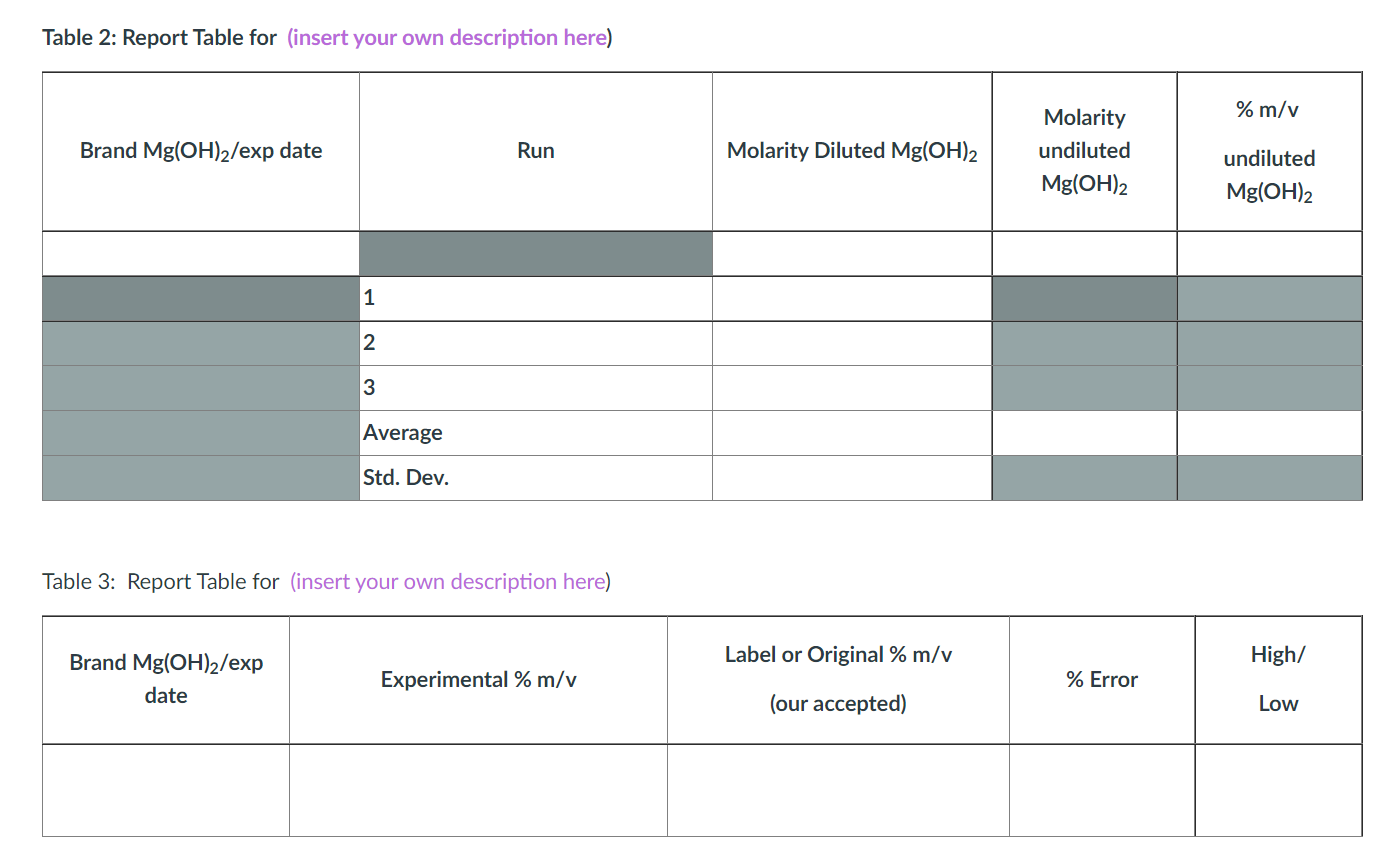
Table 2: Report Table for (insert your own description here) Brand Mg(OH)2/exp date 1 2 3 Brand Mg(OH)2/exp date Average Std. Dev. Run Table 3: Report Table for (insert your own description here) Experimental % m/v Molarity Diluted Mg(OH)2 Label or Original % m/v (our accepted) Molarity undiluted Mg(OH)2 % Error % m/v undiluted Mg(OH)2 High/ Low
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Answer Calculation 1 Molarity of Acetic Acid in Vinegar Given vv of vinegar 50 Density of vinegar 105 gmL a Calculate the molarity of acetic acid in vinegar First convert the vv of acetic acid to gmL ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


