Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Dissolution of ammonium nitrate in water Fill a 100 mL beaker half-full with water. Place your hand on the outside of the beaker to
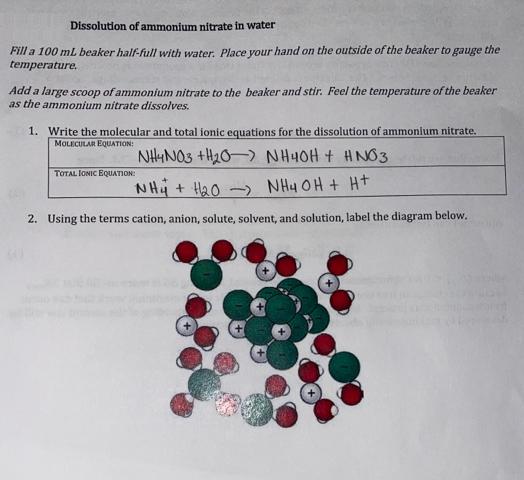

Dissolution of ammonium nitrate in water Fill a 100 mL beaker half-full with water. Place your hand on the outside of the beaker to gauge the temperature. Add a large scoop of ammonium nitrate to the beaker and stir. Feel the temperature of the beaker as the ammonium nitrate dissolves. 1. Write the molecular and total ionic equations for the dissolution of ammonium nitrate. MOLECULAR EQUATION: NH4NO3 +H20-> NH4OH + #NO3 NH4+H0 NH4OH + H+ 2. Using the terms cation, anion, solute, solvent, and solution, label the diagram below. TOTAL IONIC EQUATION: 8. The change in entropy for the reaction has to include both the AS for the salt and water. For most salts with only single charges like NaCl, the entropy change of the salt dominates. With this in mind, would your predict the entropy change of the dissolution of ammonium nitrate to be positive or negative? 9. Did the dissolution of the salt happen spontaneously? 10. For this reaction, was heat transferred from the system to the surroundings or from the surroundings to the system? 11. Was the reaction exo- or endothermic? 12. Was AHsys positive or negative? 13. Draw and label the enthalpy diagram for this reaction.
Step by Step Solution
★★★★★
3.58 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Using the terms cation anion solute solvent and solution label the diagram below The diagram is not provided in the text so I cannot label it without ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


